Table 1.
Initial discovery and condition optimizations.[a]
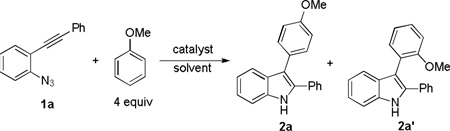 | |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | conditions | yield[b] | 2a/2a’ |
| 1 | Ph3PAuNTf2 | toluene | 60 °C, 11 h | 20%[c] | 2.4 |
| 2 | Ph3PAuNTf2 | benzene | 60 °C, 11 h | 40%[d] | 2.4 |
| 3 | Ph3PAuNTf2 | DCE | 60 °C, 11 h | 52% | 1.9 |
| 4 | Ph3PAuNTf2 | PhCl | 60 °C, 11 h | 44% | 2.1 |
| 5 | Ph3PAuNTf2 | DCE | 80 °C, 11 h | 60% | 1.9 |
| 6 | IPrAuNTf2 | DCE | 80 °C, 11 h | 81% | 4.3 |
| 7 | Cy-JohnPhosAuNTf2 | DCE | 80 °C, 11 h | 60% | 2.7 |
| 8 | t-BuXPhosAuNTf2 | DCE | 80 °C, 11 h | 66% | 8.6 |
| 9 | (ArO)3PAuNTf2[e] | DCE | 80 °C, 11 h | 73% | 2.0 |
| 10 | AuCl3 | DCE | 80 °C, 11 h | <20% | - |
| 11 | t-BuXPhosAuNTf2 | anisole (0.2 M) | 80 °C, 5 min | >95%[f] | 6.5 |
| 12 | t-BuXPhosAuNTf2 | anisole (0.2 M) | 40 °C, 1.5 h | >95% | 5.2 |
| 13 | t-BuXPhosAuNTf2 | anisole (0.2 M) | −20 °C, 12 h | >95% | 3.9 |
[1] = 0.05 M.
Estimated by 1H NMR using diethyl phthalate as the internal reference.
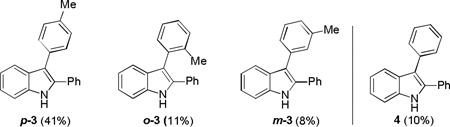
Regioisomers of 3 were formed due to reaction with solvent toluene.
2,3-diphenylindole (4) was formed in 10% yield.
Ar = 2,4-di-tert-butylphenyl.
Isolated yield: 89%.