Table 2.
The scope of o-azidoarylalkyne substrates.[a]
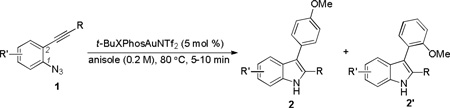 | |||||
|---|---|---|---|---|---|
| entry | 1 (R) | R’ | 2 | 2/2’ | yield[b] |
| 1 | 1b (n-butyl) | H | 2b | 7/1 | 74% |
| 2 | 1c (PhCH2CH2) | H | 2c | 5/1 | 83% |
| 3 | 1d (BnOCH2) | H | 2d | 5/1 | 51%[c] |
| 4 | 1e (cyclopropyl) | H | 2e | 8/1 | 76% |
| 5 | 1f (cyclopentyl) | H | 2f | 15/1 | 75% |
| 6 | 1g (cyclohexyl) | H | 2g | 16/1 | 82% |
| 7 | 1h (t-butyl) | H | 2h | 25/1 | 75% |
| 8 | 1i (H) | H | 2i | 3/1 | 91% |
| 9 | 1j (p-MeOPh) | H | 2j | 7/1 | 76% |
| 10 | 1k (p-MeO2CPh) | H | 2k | 7/1 | 95% |
| 11 | 1l (n-butyl) | 4,6-Me2 | 2l | 8/1 | 82% |
| 12[d] | 1m (n-butyl) | 3,5-Cl2 | 2m | 7/1 | 78% |
Vial reaction.
The combined isolated yield of 2 and 2’.
About 4% of 7 was formed.
Reaction time: 1 h.
