Table 3.
The scope of different nucleophiles.[a]
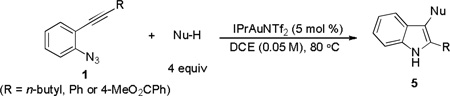 | ||||
|---|---|---|---|---|
| entry | NuH | time | 5[b] | yield[c] |
| 1 | p-xylene[d] | 5 h | 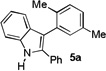 |
70% |
| 2 | 3 h |  |
78% | |
| 3 |  |
3 h | 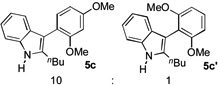 |
84% |
| 4 |  |
3 h | 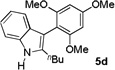 |
84% |
| 5 | 8 h | 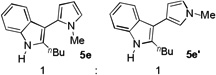 |
91% | |
| 6[e] | 12 h | 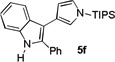 |
69%[f] | |
| 7 | 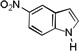 |
3 h | 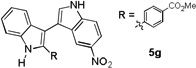 |
83% |
| 8 | 3 h | 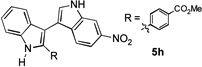 |
81% | |
| 9 | 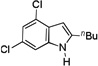 |
3 h | 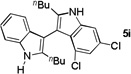 |
64% |
| 10 | 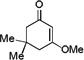 |
1 h | 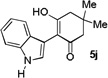 |
49% |
| 11 | n-BuOH | 15 h | 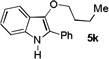 |
66% |
| 12 | n-BuOH | 12 h | 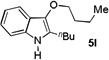 |
62% |
| 13 | allyl alcohol | 10 h | 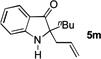 |
65% |
[1] = 0.1 M.
Regioisomers not separated. Ratio determined by 1H NMR.
Isolated yield.
Used as solvent.
10 mol % of IPrAuNTf2 used.
The regioselectivity is >9:1, and the yield is for the major isomer.
