Figure 7.
Amphipathic Helix Addition to a BAR Scaffold Is Sufficient to Mediate Membrane Scission
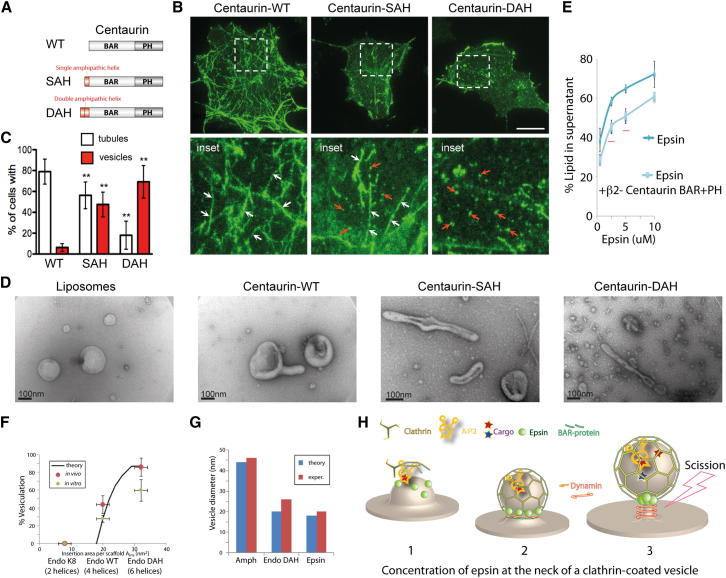
(A) Schematic representation of mutant centaurin proteins. β2-Centaurin WT BAR+PH domain had no amphipathic helices. Centaurin-SAH and centaurin-DAH had respectively a Single Amphipathic Helix or a Double Amphipathic Helix from EndoA3 at their N terminus. All constructs had a Myc-tag at the N terminus.
(B) Confocal images of COS-7 cells expressing the BAR+PH domains of centaurin-WT, centaurin-SAH, or centaurin-DAH. The first row represents the maximal projection of a 3D stack of images acquired at 0.25 μm apart. The second row displays the insets of the boxed regions. Note the tubules (white arrows) and the internal vesicles (red arrows). Bar, 10 μm.
(C) Histogram showing the percentage of transfected cells displaying internal tubules (white) and internal vesicles (red). Cells could present both. Data are the mean ± SD of >300 cells for each constructs from three independent experiments. ∗∗p < 0.001.
(D) EM of liposomes with 9 μM of the indicated proteins.
(E) Competition between epsin ENTH domain and β2-centaurin for vesiculation/tubulation of Folch liposomes. Mean ± SD for three independent experiments. Red bar: p < 0.001.
(F) Predicted percentage of vesiculated membrane by N-BAR domains covering 50% of the total membrane area as a function of the total area of inclusions per scaffold Ains. Points represent the measured values in vitro and in vivo (Figure 6) for Endo-K8 (Ains = 7 nm2), Endo-WT (Ains = 20 nm2, and Endo-DAH (Ains = 32 nm2).
(G) Predicted and measured diameters of vesicles generated as a result of membrane fission by Amph (Ains = 12 nm2, Endo-DAH (Ains = 32 nm2), and epsin ENTH domain (Ains = 6 nm2). In the computations 50% membrane coverage was used.
(H) Model of the concentration of epsin to the region of membrane scission during CCV maturation.
See also Figure S7.