Figure S5.
Membrane Vesiculation Is due to Amphipathic Helix Insertion, Related to Figure 5
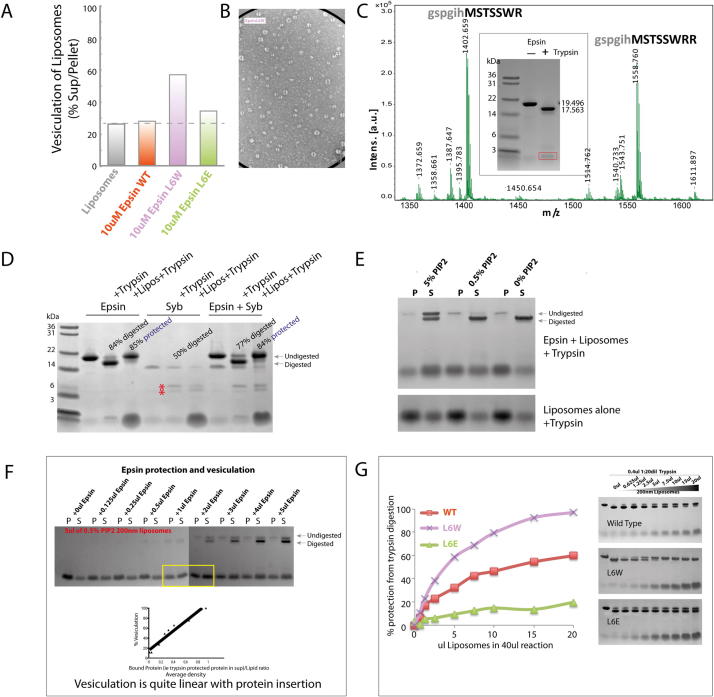
(A) At 4°C epsin vesiculation is much less efficient. After 30 min incubation with 200 nm Folch liposomes only L6W is successful in giving a partial shift of liposomes from the pellet to the supernatant in the sedimentation assay.
(B) Representative EM of liposomes after epsin L6W, used to quantitate the diameter of small vesicles shown in (A). Smaller particles (micelles or protein aggregates) were not counted.
(C) Mass spectrometry of the complete trypsin digested sample of L6W epsin ENTH domain gave 2 prominent low molecular mass peptides (red box) whose sequence shows they are digested at 2 adjacent arginines. Arg8 coordinates the 4′ phosphate of the PIP2 inositol ring, whereas Arg7 coordinates the phosophodiester linkage (Ford et al., 2002). Removal of these residues by proteolysis means that the protein no longer binds membranes (Figure 2D). The mass of the proteolysed parent ENTH domain is consistent with a further cleavage of the amphipathic helix to the next lysine. This 3 amino acid peptide was not recovered.
(D) Trypsin itself is not inhibited by membranes. The possibility that trypsin is absorbed/inhibited by membranes was tested by taking an unfolded protein (synaptobrevin) which gives distinct cleavage products (∗) and adding this to the liposome mixture. Proteins were preincubated with liposomes for 10 min before trypsin addition. Trypsin digestion of synaptobrevin is not inhibited by the addition of liposomes and may even be slightly enhanced.
(E) Epsin binding to synthetic liposomes (30% PS, 10% cholesterol, 55%–60% PC) with various PIP2 contents. Samples were subjected to 15 min incubation with trypsin to eliminate uninserted protein, showing that the limited amount of protected protein in the presence of 0.5% PIP2 was sufficient to give significant vesiculation.
(F) Increasing concentrations of epsin promote more extensive vesiculation of liposomes. After trypsin cleavage it becomes clear that, at higher concentrations, most of the added epsin is not bound/protected by the liposomes and so does not contribute to vesiculation. When vesiculation is plotted versus the bound/protected protein, vesiculation is linear relative to inserted epsin protein. The yellow box indicates the point of approximately 50% vesiculation where there is maximally 10% membrane coverage by epsin (assuming that 6ul epsin is giving saturation).
(G) Membrane binding of epsin ENTH domain and mutants. It is not easy to assess the amount of epsin bound to membrane by the traditional sedimentation assay as the protein causes a shift in the liposomes sedimentation pattern. However given that amphipathic helix insertion is the major event one wants to monitor and is a reflection of binding, we can use trypsin sensitivity of the protein as a measure of insertion. At room temperature for 30 min L6W mutant binds better than WT protein, which binds better than L6E mutant protein.