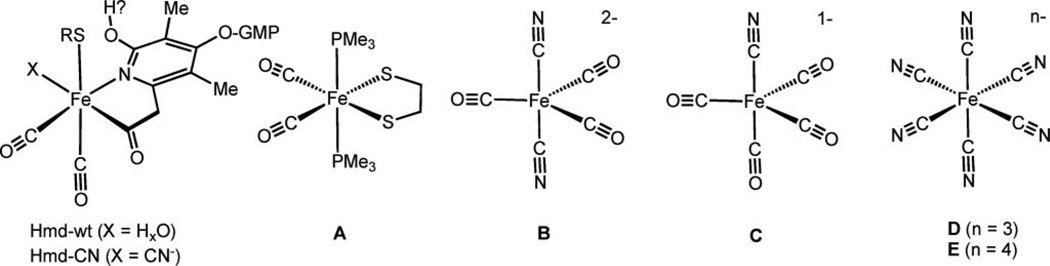
Fig. 1.
On the left: structure of the [Fe]-hydrogenase octahedral metal binding site as modelled by EXAFS analysis.1 The iron is coordinated to: the Cys176-sulfur, two CO and the pyridinol-sp2-hybridized nitrogen and the acyl-carbon of the cofactor. An “unknown” donor, here represented as X, is associated to the iron trans to the acyl carbon. This is considered the vacant position ready for H2 binding. The model complexes are represented in A–E: Fe(ii)(edt)(CO)2(PMe3)2 (A); K2[Fe(0)(CN)2(CO)3] (B); K[Fe(0)(CN)(CO)4] (C); K3[Fe(iii)(CN)6] (D); K4[Fe(ii)(CN)6] (E).