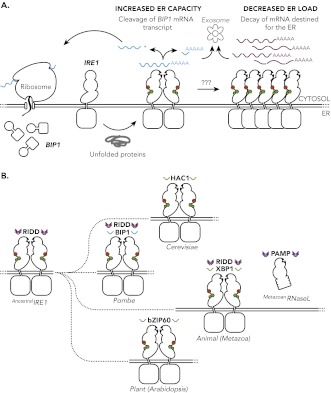
Figure 1.
The unfolded protein response in S. pombe and other species. (A) The accumulation unfolded proteins in the endoplasmic reticulum (ER) of S. pombe leads to activation of IRE1 (presumably by nucleotide binding (green), auto-phosphorylation (red) and the formation of dimers), which is turn leads to the cleavage of mRNAs in the cytosol. The subsequent degradation of the cleaved mRNAs (known as RIDD) and explusion from the cell (via the exosome) reduced the protein-folding load on the ER. However, as described in the text, the mRNA that encodes for the molecular chaperone Bip1 escapes this fate: although it is cleaved at the 3′ untranslated region (shown by the asterisk), the 5′ Bip1 mRNA fragment is first stabilized and then translated into Bip1 by the ribosome. The enhanced production of Bip1 helps to increase the protein-folding capacity of the ER. The same IRE1 dimer can perform both Bip1 mRNA cleavage and RIDD: however, larger IRE1 oligomers (right) can also perform RIDD in a second level of the unfolded protein response. (B) Ancestral IRE1 may have possessed RIDD activity prior to the evolution of the yeasts (top), animals (middle) and plants (bottom). This vestigial RIDD function is not evident in budding yeast (S. cerevisiae), and it is not known if it exists in plants (Arabidopsis), but it is evident in the animals (metazoans). However, in all three cases IRE1 exerts transcriptional control by splicing mRNA to regulate the expression of various transcription factors (HAC1, XBP1 and bZIP60). The unfolded protein response in fusion yeast (S. Pombe) is different in that it involves RIDD and the direct post-transcriptional stabilisation of the molecular chaperone Bip1. RNase L is a distant relative of IRE1 that triggers mRNA decay in mammals in a way that is not dissimilar to RIDD.