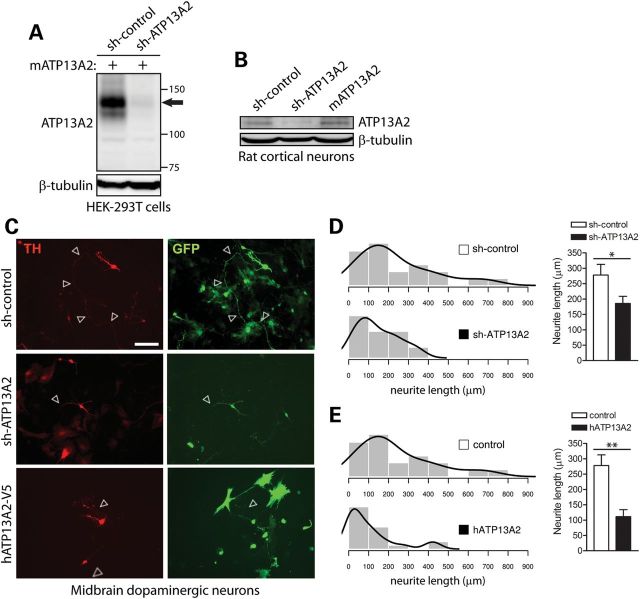
Figure 4.
Modulation of ATP13A2 expression impairs neurite outgrowth of midbrain dopaminergic neurons. (A) Soluble extracts from HEK-293T cells transiently co-expressing untagged mouse ATP13A2 and rodent-specific ATP13A2 (sh-ATP13A2) or non-silencing control (sh-control) shRNA plasmids were probed with an antibody to ATP13A2 (LMNR1) to demonstrate shRNA-mediated knockdown of ATP13A2. β-tubulin indicates equivalent protein loading. (B) Soluble extracts from rat primary cortical neurons transiently expressing control or ATP13A2-specific shRNAs, or untagged mouse ATP13A2, were probed with antibodies to ATP13A2 (LMNR1) or β-tubulin as a control for protein loading. ATP13A2-specific shRNA reduces the levels of endogenous ATP13A2 compared with control shRNA, whereas overexpression of mouse ATP13A2 is also detected. (C) Rat primary midbrain dopaminergic neurons co-transfected at DIV 3 with shRNA (control or ATP13A2-specific) or V5-tagged human ATP13A2 (or control empty vector) and GFP plasmids at a 10:1 molar ratio. Cultures were fixed at DIV 7. Representative fluorescent micrographs reveal the co-labeling of dopaminergic neurons with GFP and TH for each condition. Axonal processes are indicated by arrows. Scale bar: 100 µm. (D and E) Quantitative analysis of the length of GFP+/TH+ dopaminergic neurites reveals a significant shortening of axonal processes due to (D) the shRNA-mediated silencing of ATP13A2 or (E) the overexpression of human ATP13A2, compared with control neurons (sh-control or empty vector). Bars represent the mean ± SEM length of TH+ neurites in micrometers (n = 39–42 neurons for shRNAs or n = 28–39 neurons for hATP13A2) sampled across four independent cultures. Histograms indicate the mean frequency distribution of neurite length for each condition (n = 4 experiments). *P < 0.05 or **P < 0.01 compared with control plasmids assessed by unpaired Student's t-test.