Table 2.
Morpholine Synthesis Scopea
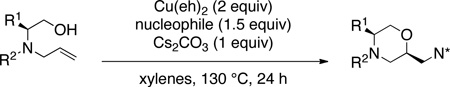 | ||||
|---|---|---|---|---|
| entry | substrate | nucleophile | product (dr)b | yieldc |
| 1 | 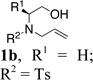 |
TsNH2 | 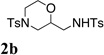 |
80% |
| 2 | 1c, R1 = CH3, R2 = Ts | TsNH2 | 73% | |
| 3 | 1d, R1 = i-Pr, R2 = Ts | TsNH2 | 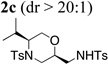 |
82% |
| 4 | 1e, R1 = CH2OTBS, R2 = Ts | TsNH2 | 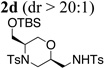 |
72% |
| 5 | 1f, R1 = CH2SBn, R2= Ts | TsNH2 | 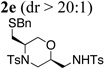 |
42% |
| 6 | 1g, R1 = Bn,R2 = Ns | TsNH2 | 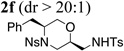 |
83% |
| 7 | 1h, R1 = Bn,R2 = Ms | TsNH2 | 73% | |
| 8 | 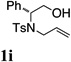 |
TsNH2 | 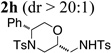 |
67% |
| 9 | 1a, R1 = Bn; R2 = Ts | NsNH2 | 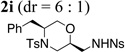 |
82% |
| 10 | 1a | PMBSNH2 | 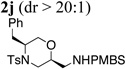 |
82% |
| 11d | 1a | MsNH2 | 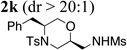 |
43% |
| 12 | 1a | SESNH2 | 98% | |
| 13d | 1a | PhC(O)NH2 | 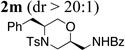 |
87% |
| 14d | 1a | NaN3 | 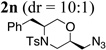 |
53% |
| 15e | 1c | PhC(O)NH2 | 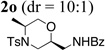 |
73% |
| 16f | 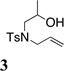 |
TsNH2 | 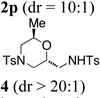 |
52% |
Same conditions as Table 1, entry 4 unless otherwise noted.
Diastereoselectivity obtained by analysis of the crude 1H NMR spectra.
Isolated yields.
Reaction was run using 4 equiv Cu(eh)2.
Reaction was run using 3 equiv of Cu(eh)2.
Reaction was run at 150 °C.
