Fig. 4.
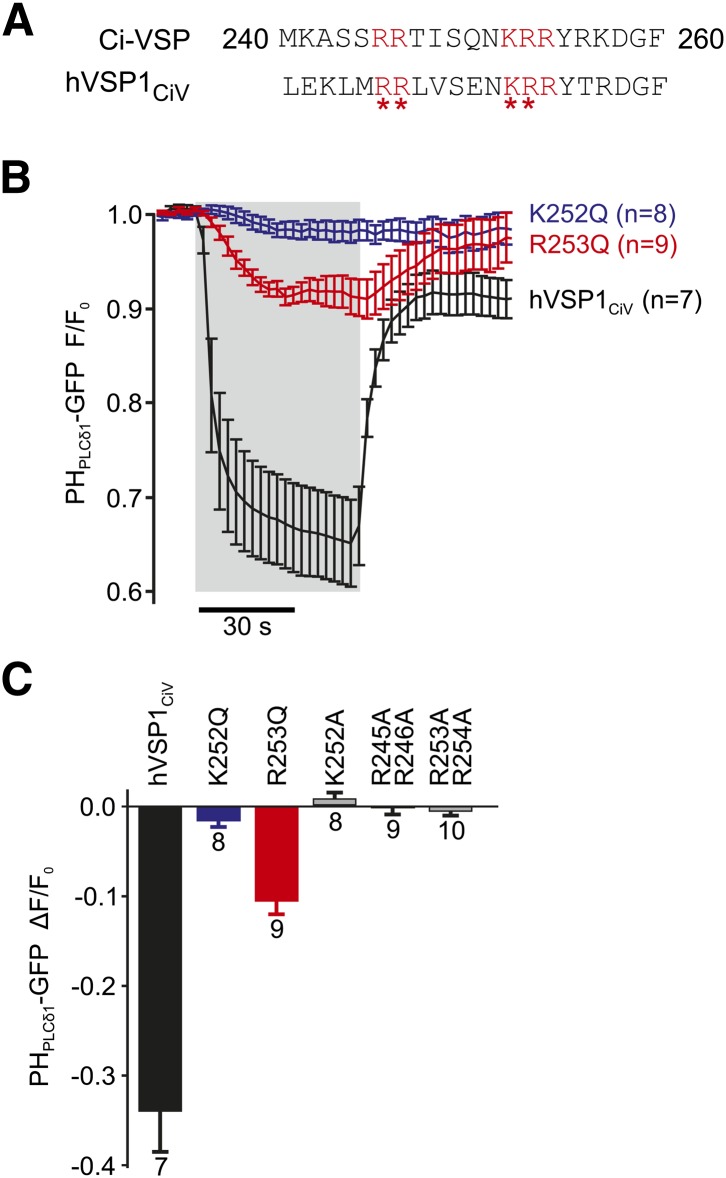
Functional coupling of the enzymatic domain to the VSD is mediated by the PBM. A: Critical basic residues (red) are conserved between the PBMs of Ci-VSP and hVSP1. Asterisks indicate positions mutated to glutamine or alanine. B: Charge-neutralizing mutations K252Q and R253Q in the PBM of hVSP1 greatly reduce voltage-dependent activation of hVSP1CiV. Experiments were performed as in (Fig 1A, B), and data from cells expressing wt hVSP1CiV are replotted from Fig. 1B for comparison. Depolarization to 80 mV is indicated by shaded area [N = 8 (K252Q) or 9 (R253Q) cells]. C: Average depolarization-activated enzymatic activity for the PBM mutants indicated. Activity is quantified as the degree of dissociation of PHPLCδ1-GFP from the membrane, measured as in B.