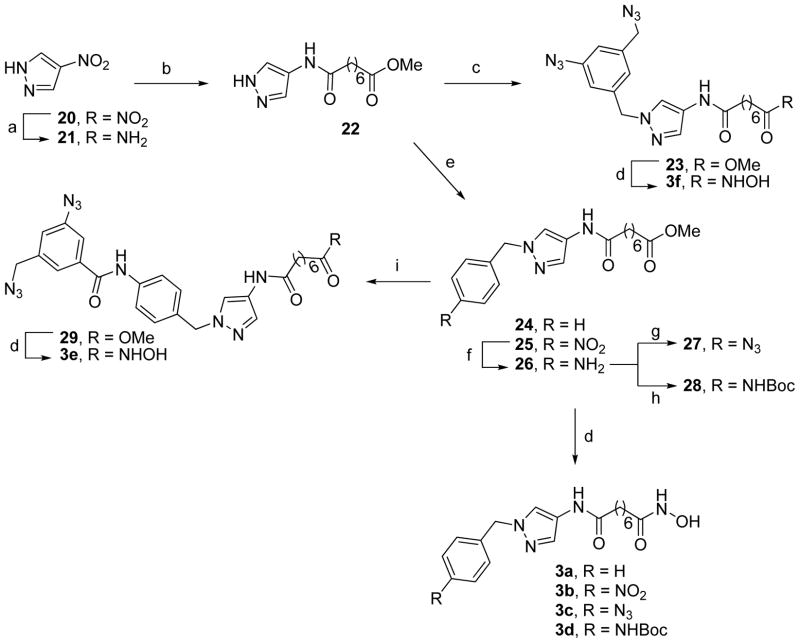
Scheme 3.
Synthesis of 3a–3fa
aReagents and conditions: (a) H2, Pd/C, MeOH, overnight; (b) monometyl suberate, EDC, HOBt, DIPEA, CH2Cl2, 0 °C–rt, 6 h; (c) 8, K2CO3, acetone, reflux, 6 h; (d) NH2OH, KOH, 0 °C–rt, 3 h; (e) BnBr or 4-nitrobenzyl bromide, NaH, DMF, 0 °C–rt, 4 h; (f) SnCl2·2H2O, MeOH, reflux, 2 h; (g) NaNO2, AcOH–H2O (9:1), 0 °C, 10 min, NaN3, 0 °C–rt, 1 h; (h) (Boc)2O, Et3N, CH2Cl2, rt, 4 h; (i) 12, EDC, HOBt, DIPEA, CH2Cl2, 0 °C–rt, 6 h.