Table 2.
HDAC3 and HDAC8 isoform inhibitory activity (IC50, nM) of pyrazole-based compounds 3a–3f.
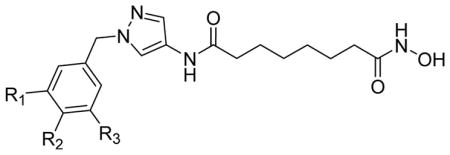
| ||||
|---|---|---|---|---|
| compd | Substituents R1, R2, R3 | IC50 ± SD (nM) | HDAC8/HDAC3a | |
| HDAC3 | HDAC8 | |||
| 3a | R1, R2, R3 = H | 44 ± 5.8 | 76 ± 5.0 | 1.7 |
| 3b | R2 = NO2, R1, R3 = H | 59 ± 1.0 | 82 ± 9.0 | 1.4 |
| 3c | R2 = N3 R1, R3 = H | 22 ± 1.3 | 28 ± 3.0 | 1.3 |
| 3d | R2 = NHBoc R1, R3 = H | 191 ± 18 | 147 ± 15 | 0.8 |
| 3e |
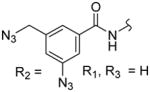
|
432 ± 52 | 487 ± 80 | 1.1 |
| 3f | R1 = CH2N3 R2 = H R3 = N3 | 128 ± 9.8 | 17 ± 3.0 | 0.13 |
ratio of IC50 (nM)
