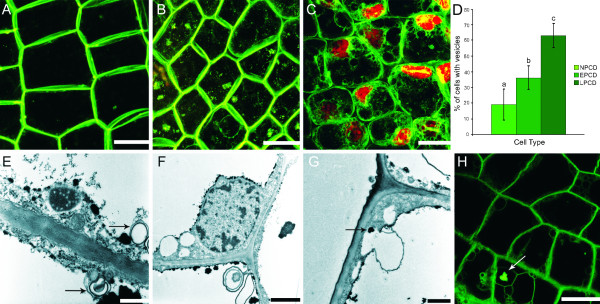
Figure 4.
Presence of autophagic vesicles throughout lace plant PCD. (A) Non-PCD (NPCD) stage cells co-stained with FM1-43 (green), a dye that localizes to phospholipid bilayers, and PI (red), a dye that localizes to the nucleus. Staining of the PM and possibly the tonoplast, in addition to several small round organelles can be seen. The absence of PI staining indicated healthy nuclei. (B) EPCD stage cells co-stained with FM1-43 and PI. Note stained PMs, some tonoplast staining, in addition to several autophagic vesicles. Organelle aggregates were present and stained with FM1-43. Again recognize the absence of PI staining, indicating healthy nuclei. (C) LPCD stage cells co-stained with FM1-43 and PI. Note PM, tonoplast and organelle staining as well as the multiple, variable sized vesicles. Most nuclei in LPCD cells are stained with PI, indicating non-viable nuclei (D) Percentage of cells containing vesicles by stage. NPCD, EPCD and LPCD stages had 22, 38 and 66 percent of their cells containing vesicles, respectively. Bars with different letters differed significantly (P < 0.05). Error bars represent standard error of a minimum of 120 cells per category. (E) TEM micrograph of a late stage lace plant leaf. Double-membraned vesicular structures, some of which contain organelle material can be seen (marked by black arrow). (F) TEM micrograph of a late stage lace plant cell. Note multiple vesicles, including one double membraned vesicle containing organelle material. (G) TEM image of late stage PCD cell depicting invagination of the tonoplast, possibly indicating micro-autophagy. Electron dense material in the invagination that is possibly composed of organelles can be seen (marked by black arrow). (H) LPCD stage cell stained with FM1-43. Note the stained PM, organelles and vesicles. White arrow points to an aggregate that appears to be within a small vacuole. Scale bars: A = 50 μm, B-C = 100 μm, E = 0.4 μm, F = 1 μm, G = 0.8 μm, H = 100 μm.