Abstract
Immunologically-silent microglial phagocytosis of apoptotic cells and cellular debris is critical for CNS homeostasis and innate immune balance. The beneficial and detrimental effects of microglial phagocytosis on neurons remain controversial. Phagocytosis ligands are the key to selecting extracellular cargos, initiating the engulfment process, defining phagocyte functional roles and regulating phagocyte activities with therapeutic potentials. Here we characterized tubby as a new ligand to regulate microglial phagocytosis through MerTK receptor, which is well known for its immunosuppressive signaling. Tubby at 0.1 nM significantly induced microglial phagocytosis of apoptotic cells with a maximal activity at 10 nM. Tubby activated MerTK with receptor autophosphorylation in a similar dose range. Excessive soluble MerTK extracellular domain blocked tubby-mediated microglial phagocytosis of plasma membrane vesicles as cellular debris. Immunocytochemistry revealed that the ingested cargos were co-localized with MerTK-dependent non-muscle myosin II, whose rearrangement is necessary for cargo engulfment. Phagosome biomarker Rab7 was colocalized with cargos, suggesting that internalized cargos were targeted to phagocytic pathway. Tubby stimulated phagocytosis by neonatal and aged microglia with similar activities, but not by MerTK−/− microglia. These results suggest that tubby is a ligand to facilitate microglial phagocytosis through MerTK for the maintenance of CNS homeostasis.
Keywords: Microglia, phagocytosis, tubby, MerTK, phagocytosis ligand, eat-me signal
Introduction
Anti-inflammatory phagocytosis of autologous apoptotic cells, also called engulfment or efferocytosis, is distinctively different from pro-inflammatory phagocytosis of foreign pathogens via different ligands and receptors (Erwig and Henson, 2007; Napoli and Neumann, 2009). Microglial phagocytosis of apoptotic cells or cellular debris is well recognized for the maintenance of CNS homeostasis and innate immune balance (Napoli and Neumann, 2009; Neumann et al., 2009), but has controversial effects. During neurogenesis, excess neurons are generated, deleted through apoptosis and removed by microglial phagocytosis (Schlegelmilch et al., 2011). Microglial phagocytosis also plays an important role in axon pruning for the restructuring of neuronal connections (Mallat et al., 2005; Neumann et al., 2009). The prompt and efficient clearance of apoptotic cells by microglial phagocytosis is essential to prevent dying cells from releasing intracellular contents and triggering inflammation and/or autoimmune response (Nagata et al., 2010). However, inappropriate microglial phagocytosis may also result in phagocytosis-mediated killing of stressed but viable neurons during neuroinflammation, and blockade of ligand-dependent phagocytosis can preserve live neurons (Fricker et al., 2012).
Molecular phagocyte biology, including phagocytosis ligands, receptors and intracellular signaling molecules (Li, 2012), is the key to understanding beneficial and detrimental roles of microglial phagocytosis but is largely undefined. For example, triggering receptor expressed by myeloid cells-2 (TREM2) is a microglial/macrophage phagocytic receptor with unknown ligand(s) (Hsieh et al., 2009). Mutations of TREM2 cause Nasu-Hakola disease, a chronic and fatal neurodegenerative disease with defect in microglial phagocytosis (Neumann and Takahashi, 2007). In contrast, mutations of Mer receptor tyrosine kinase (MerTK), a well-characterized phagocytic receptor expressed in many phagocytes, including retinal pigment epithelial (RPE) and microglial cells, cause retinal degeneration without CNS manifestations (Gal et al., 2000). The molecular mechanisms for the tissue-specific degenerations associated with MerTK or TREM2 mutations remain elusive. MerTK is an important phagocytic receptor for immunosuppressive signaling (Lemke and Rothlin, 2008; Ravichandran and Lorenz, 2007). Its ligands in CNS are poorly defined but will provide insights into functional roles of MerTK signaling pathway and the controversial effects of microglial phagocytosis.
Gas6 was the only MerTK-specific ligand characterized for microglial phagocytosis (Grommes et al., 2008). We recently identified tubby and tubby-like protein 1 (Tulp1) as phagocytosis ligands or eat-me signals for RPE via MerTK (Caberoy et al., 2010a; Caberoy et al., 2010c). While Tulp1 is highly expressed in the retina, tubby is expressed in embryonic and adult brain as well as the retina (Ikeda et al., 1999; Sahly et al., 1998). Here we demonstrate that tubby activated MerTK in microglia with receptor phosphorylation and facilitated phagocytic clearance of apoptotic cells or cellular debris in a similar concentration-dependent manner. This was verified by MerTK blockade and tubby-induced MerTK-dependent non-muscle myosin II (NMMII) rearrangement. Ingested cargos through tubby-MerTK-NMMII pathway were targeted to phagosomes and co-localized with phagosome biomarker Rab7. Tubby stimulated phagocytosis by BV-2 microglial cell line and primary microglia, but not by MerTK−/− microglia. Neonatal and aged primary microglia showed similar activities for tubby-mediated phagocytosis. These results suggest that tubby is a MerTK-specific ligand for regulating microglial phagocytosis.
2. Materials and methods
2.1. Animals
C57BL/6 mice and MerTK−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME), housed and bred under standard conditions in groups of 3–5 per cage.
2.2. Microglia
BV-2 microglial cell line was cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% FBS and 2 mM L-glutamine (Caberoy et al., 2009). Primary microglia were prepared as previously described with following modifications (Moussaud and Draheim, 2010; Njie et al., 2010). Mice at postnatal day 4–8 or 15 months of age were euthanized. The brains were harvested, washed with phosphate-buffered saline (PBS), minced and digested with 0.1% collagenase (Invitrogen, Carlsbad, CA) in DMEM/F12 medium at 37°C for 1 h with shaking. The cells were dispersed by pipetting, filtered through a 70-µm sterile nylon mesh (BD Biosciences, San Jose, CA), and purified by centrifugation with Percoll discontinuous gradient (35%/75%) (Njie et al., 2010). The cells at the interface of low and high Percoll were collected, washed and cultured in DMEM/F12 medium supplemented with 10% FBS, GlutaMAX™ (Invitrogen) and murine recombinant granulocyte-macrophage colonystimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN) (Moussaud and Draheim, 2010). The medium was replaced every 3–4 days. The floating microglia at the medium surface were collected from day 14–60 and analyzed for purity by flow cytometry using anti-CD11b monoclonal antibody (Biolegend, San Diego, CA) (Caberoy et al., 2010a). The primary microglia were cultured for 3 days in the complete medium without GM-CSF before experiments.
2.3. Apoptotic cells
Jurkat cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 2 mM L-glutamine. The cells were treated with staurosporine (1 µg/ml) for 3 h, as described (Caberoy et al., 2010c). After washing, the cells were stained with Annexin V and propidium iodide and analyzed for apoptosis by flow cytometry to confirm apoptosis (Caberoy et al., 2010c). The results indicated that ~98% of treated cells were apoptotic. Control cells were ~96% healthy. The cells were labeled with pHrodo™ succinimidyl ester (Invitrogen), as described (Caberoy et al., 2012; Miksa et al., 2009). Briefly, apoptotic or healthy cells (1×106 cells/ml) were incubated with pHrodo (20 ng/ml in PBS, stock 1 mg/ml in DMSO) for 30 min at room temperature. Labeled cells were washed twice with DMEM complete medium containing 10% FBS.
2.4. Membrane vesicles
Plasma membrane vesicles were prepared from Neuro-2a cells by sucrose gradient centrifugation, as described (Caberoy et al., 2010a). Briefly, Neuro-2a cells at 80% confluence were harvested, homogenized and purified by sucrose gradient (20%/27%) centrifugation at 105,000×g for 1 h. Plasma membrane vesicles at the sucrose interface were collected, washed twice and resuspended in PBS. Purified plasma membrane vesicles were labeled with pHrodo as above and used for phagocytosis assay.
2.5. Phagocytosis assay
Glutathione S-transferase (GST)-tubby and GST control were prepared, as previously described (Caberoy et al., 2010b). FLAG-tagged tubby (FLAG-tubby) and mock FLAG peptide were expressed in HEK293 cells and purified with anti-FLAG M2 monoclonal antibody affinity column (Caberoy et al., 2010c). BV-2 cells or primary microglia were seeded in 2-chamber tissue culture slides (Nunc, Rochester, NY) at ~1×105 cells/chamber and cultured for overnight. Labeled membrane vesicles (~50 µg protein/chamber) or Jurkat cells (3×105 cells/chamber) were added to the microglia in the presence or absence of purified tubby or mock control at the indicated concentrations for phagocytosis at 37°C for 3 h. Alternatively, pHrodo-labeled membrane vesicles or Jurkat cells were preincubated with GST-tubby or GST (10 nM) for 30 min, washed to remove the unbound protein, and incubated with microglia for phagocytosis in the presence or absence of excess MerFc (MerTK extracellular domain fused to human IgG1 Fc domain, 2.5 µg/ml, R&D Systems). After washing, microglia were fixed with 4% buffered formalin, and all intracellular fluorescent signals were analyzed by confocal microscopy, as described (Caberoy et al., 2010a; Caberoy et al., 2010c). Relative fluorescence intensity per microglia was measured using Leica Application Suite software by manually tracing the outline of individual phagocytes under bright field channel with the corresponding fluorescence quantified. More than 100 cells per group were analyzed.
2.6. Immunocytochemistry
Microglia with phagocytosed pHrodo-labeled cargos were fixed with buffered formalin, permeabilized with PBS buffer containing 1% Triton X-100, immunostained with anti-Rab7 (Abcam, Cambridge, MA) or anti-NMMII-A heavy chain antibodies (Sigma, St. Louis, MO), followed by FITC-labeled secondary antibodies. The nuclei were visualized by DAPI. The intracellular fluorescence signals were analyzed by confocal microscopy.
2.7. MerTK activation
MerTK phosphorylation was analyzed, as described (Caberoy et al., 2010c). Briefly, microglia were cultured in 293 SFM II medium (Invitrogen) for 2 h to reduce the background of MerTK activation. The cells were incubated with purified GST-tubby or GST control at the indicated concentrations for 30 min at 37°C. After washing, the cells were lysed (Sather et al., 2007). The lysates were incubated with anti-MerTK antibodies (FabGennix, Frisco, TX), followed with protein A resin. The beads were precipitated by centrifugation, washed and analyzed by Western blot using anti-phospho-MerTK (FabGennix) or anti-MerTK antibodies, followed with HRP-conjugated secondary antibodies and chemiluminescence detection.
2.8. Statistical analysis
All experiments were repeated independently at least 3 times. Data were expressed as means ± SEM and analyzed by Student’s t-test or Tukey-Kramer multiple comparisons test using GraphPad Instat software. Data were considered significant when p < 0.05.
3. Results
3.1. Tubby facilitates microglial phagocytosis of apoptotic cells
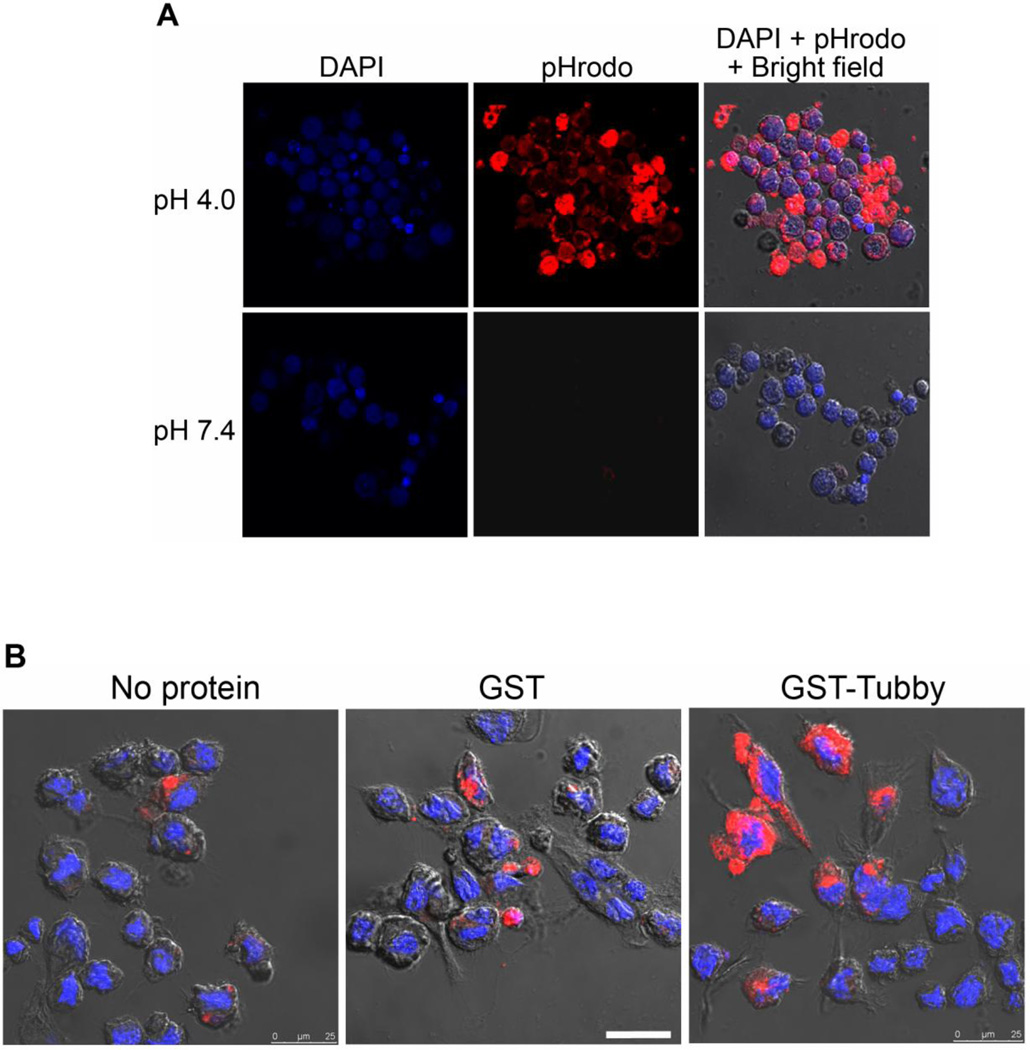
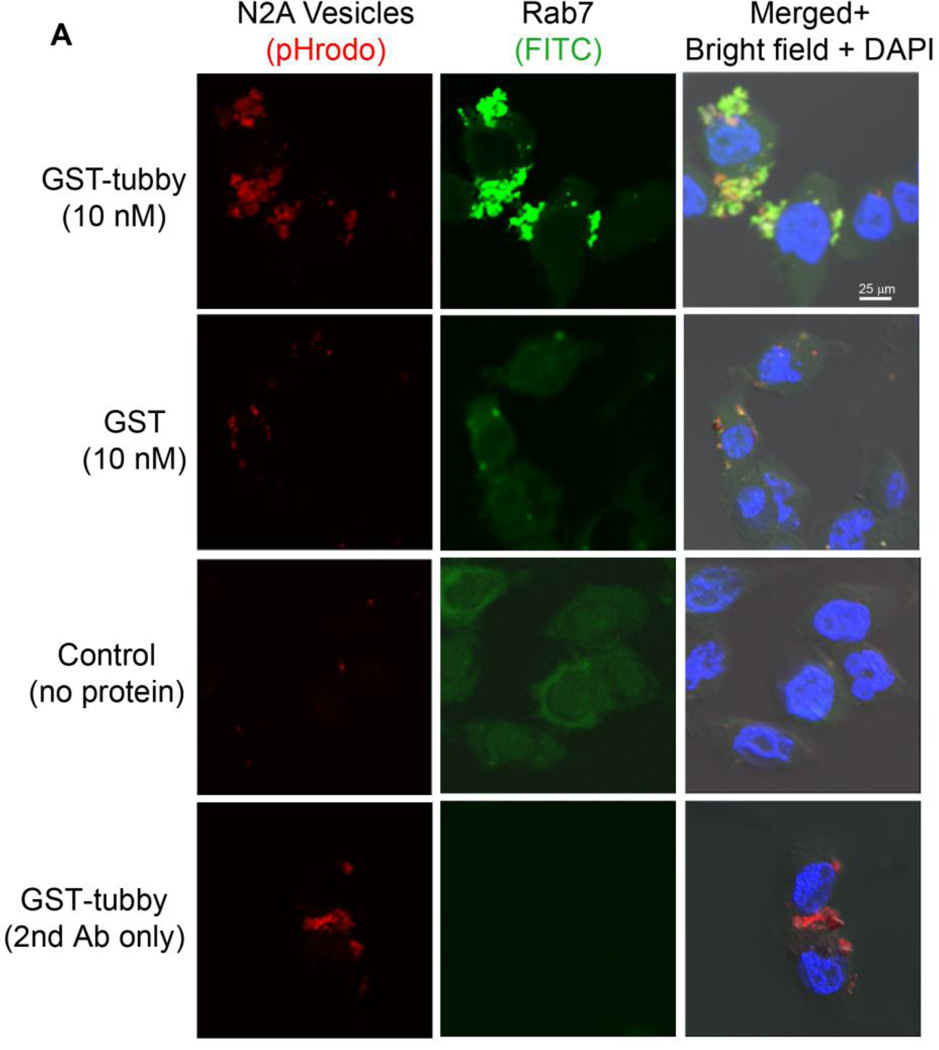
To reliably distinguish between internalized and surface-bound cargos, we labeled apoptotic and healthy Jurkat cells with pHrodo dye for microglial phagocytosis assay. pHrodo is a fluorogenic dye with no fluorescence at neutral pH but drastically increases in fluorescence intensity in an acidic environment (Fig. 1A). When the labeled cargos were internalized and targeted to acidic phagosomes, the dye was activated and generated red fluorescence (Caberoy et al., 2012; Miksa et al., 2009). Coupled with confocal microscopy, pHrodo enables reliable distinction between ingested and uningested cargos. We analyzed the phagocytosis of pHrodo labeled Jurkat cells by BV-2 microglial cells. The results showed that purified GST-tubby stimulated microglial phagocytosis in a dose-dependent manner (Fig. 1B,C). Tubby induced significantly higher phagocytosis of apoptotic cells at ~0.1 nM with a maximal activity at ~10 nM. Interestingly, higher concentration of tubby (200 nM) resulted in reduced activity to stimulate microglial phagocytosis. Control GST showed minimal activity.
Fig. 1.
Microglial phagocytosis. (A) pHrodo is a pH-sensitive fluorescence dye. Jurkat cells were harvested, labeled with pHrodo, treated with different pH buffer and analyzed by confocal microscopy. (B) BV-2 cell phagocytosis in the presence or absence of tubby. pHrodo-labeled apoptotic or healthy Jurkat cells were incubated with BV-2 cells in the presence of purified GST-tubby or GST control (10 nM) for phagocytosis for 3 h. Phagocytosed pHrodo-Jurkat cells were analyzed by confocal microscopy. The DAPI signal for nuclei and pHrodo signal for ingested cargos from the same z-stack are superimposed with the cognate bright field to reveal the phagocytosed cargos. Only apoptotic Jurkat cells are shown here. Bar = 25 µm. (C) Relative fluorescence intensity per cell in (B) was quantified in more than 100 cells per group (± SEM, n>100; Tukey-Kramer test).
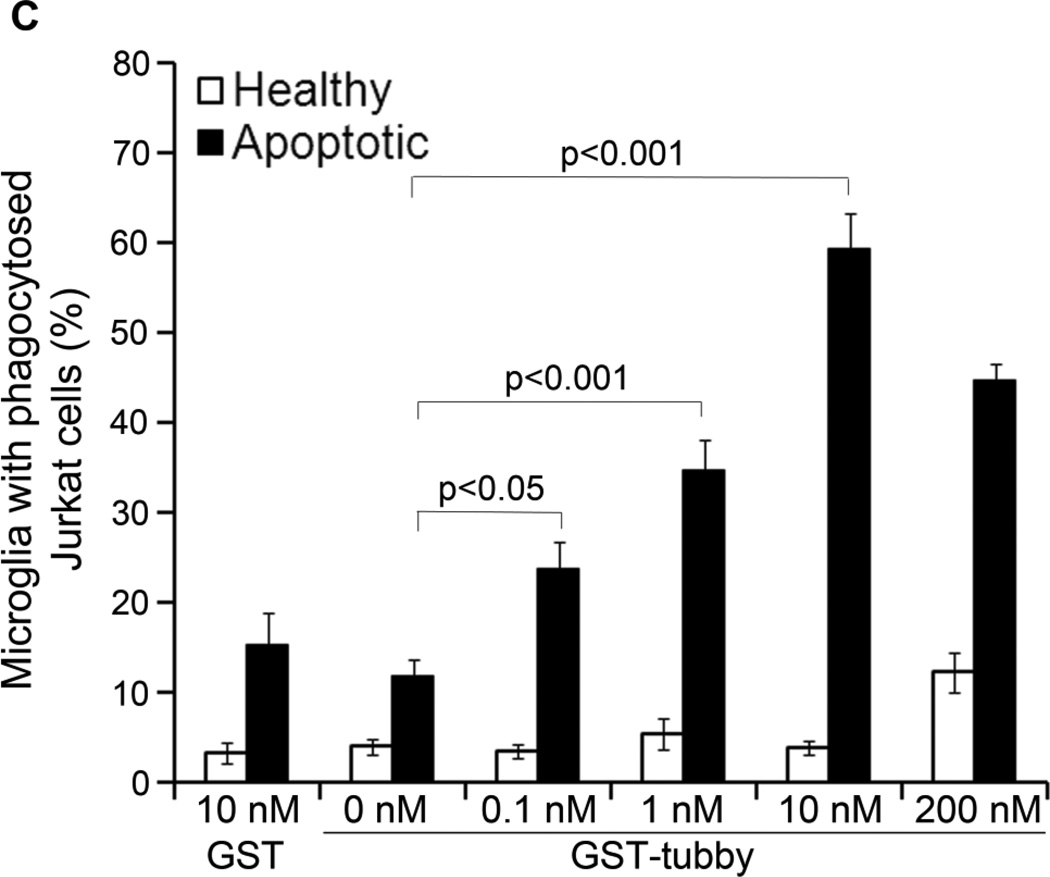
3.2. Tubby directs the ingested cargos to phagosomes
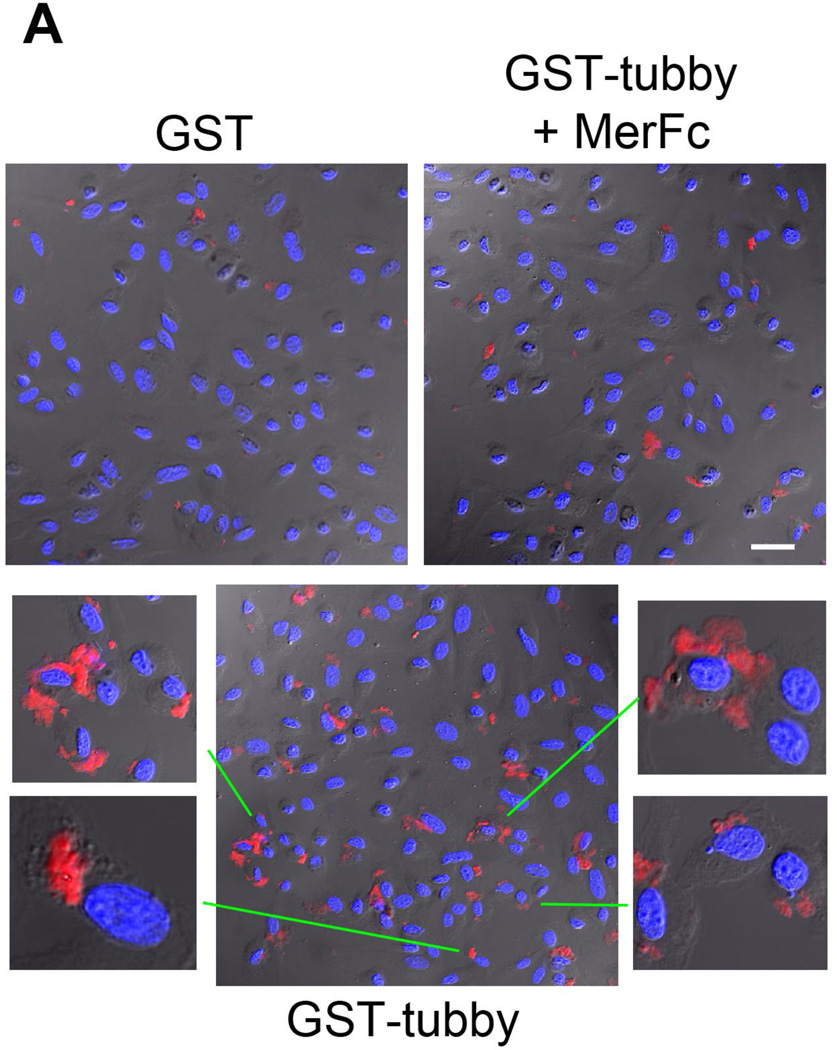
To further validate that ingestion of pHrodo-labeled cargos via the phagocytic pathway, we prepared plasma membrane from Neuro-2a cells, used the pHrodo-labeled membrane vesicles as cellular debris for microglial phagocytosis, and analyzed the co-localization of the internalized vesicles with phagosome biomarker Rab7 by immunocytochemistry. The results showed that pHrodo signals of internalized cargos were co-localized with Rab7 (Fig. 2A). To eliminate the possible influence of GST, we purified recombinant FLAG-tubby and analyzed its activity to stimulate microglial phagocytosis. The results showed that tubby with different tags have the same functional activity (Fig. 2B,C).
Fig. 2.
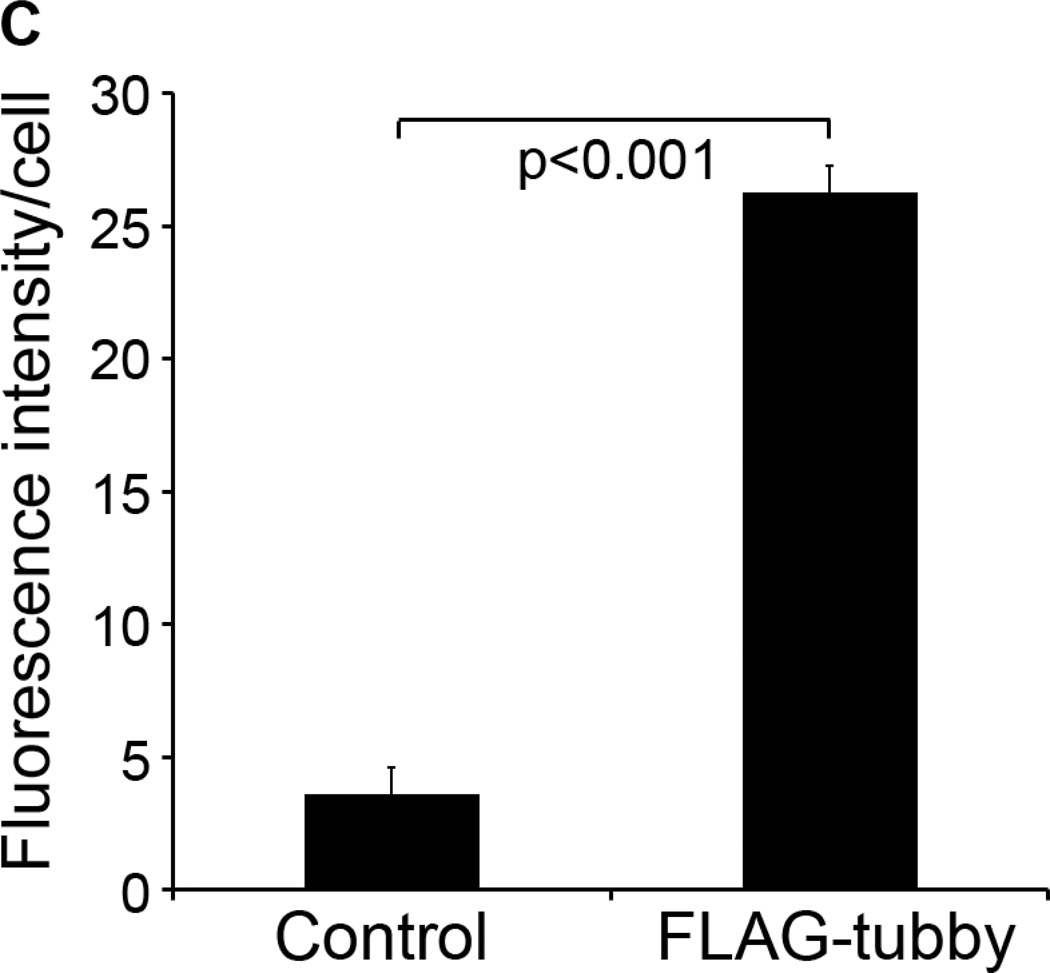
Tubby-mediated microglial engulfment targets to phagosomes. (A) Microglial phagocytosis stimulated by GST-tubby. Plasma membrane vesicles were prepared from Neuro-2a (N2a) cells, labeled with pHrodo and incubated with BV-2 microglia in the presence of GST-tubby or GST (10 nM) for phagocytosis. Phagosome marker Rab7 was detected using anti-Rab7 antibodies and FITC-labeled secondary antibodies, and analyzed by confocal microscopy. pHrodo signals and FITC signals are co-localized and superimposed with DAPI signals and the cognate bright field. Bar = 25 µm. (B) Microglial phagocytosis with FLAG-tubby. Phagocytosis assay was performed with purified FLAG-tubby or mock control as in (A). Bar = 10 µm. (C) Relative fluorescence intensity per cell in (B) was quantified in more than 100 cells per group (± SEM, n>100; t-test).
3.3. Tubby activates MerTK in microglia
Tubby was identified as a novel MerTK ligand in our recent study in RPE (Caberoy et al., 2010a). MerTK is expressed in many other phagocytes, including macrophages and microglia (Caberoy et al., 2012; Grommes et al., 2008), and ligand-induced MerTK activation results in the receptor autophosphorylation (Caberoy et al., 2012; Caberoy et al., 2010c). We demonstrated microglial MerTK activation induced by tubby (Fig. 3A), suggesting that MerTK is an important phagocytic receptor in microglial phagocytosis as well. It is interesting that tubby induced a similar concentration-dependent induction of MerTK phosphorylation with minimal and maximal concentrations at 0.1 nM and 10 nM, respectively (Fig. 3A). It is worth noting that tubby is more active to simulate MerTK phosphorylation than Gal-3, another new MerTK ligand recently identified by our group (Caberoy et al., 2012). The minimal concentration to induce MerTK phosphorylation was 0.1 nM for tubby (Fig. 3A) and 50 nM for Gal-3 (Caberoy et al., 2012). However, actual biological activity of both proteins depends on their local extracellular concentrations in in vivo settings and is yet to be defined.
Fig. 3.
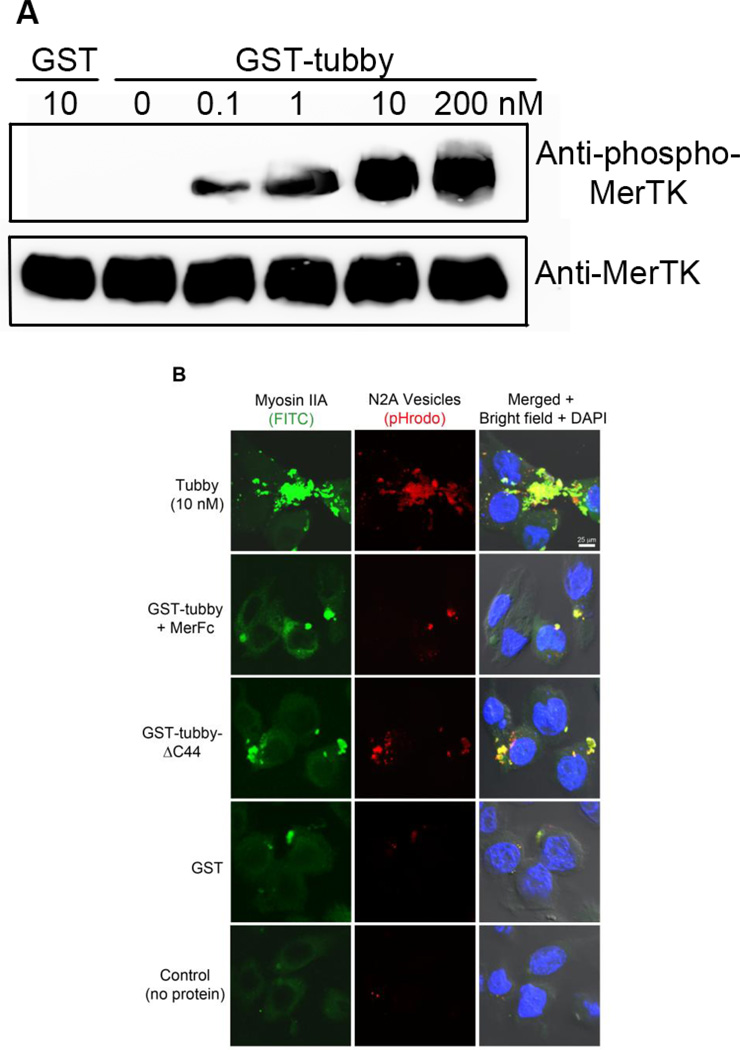
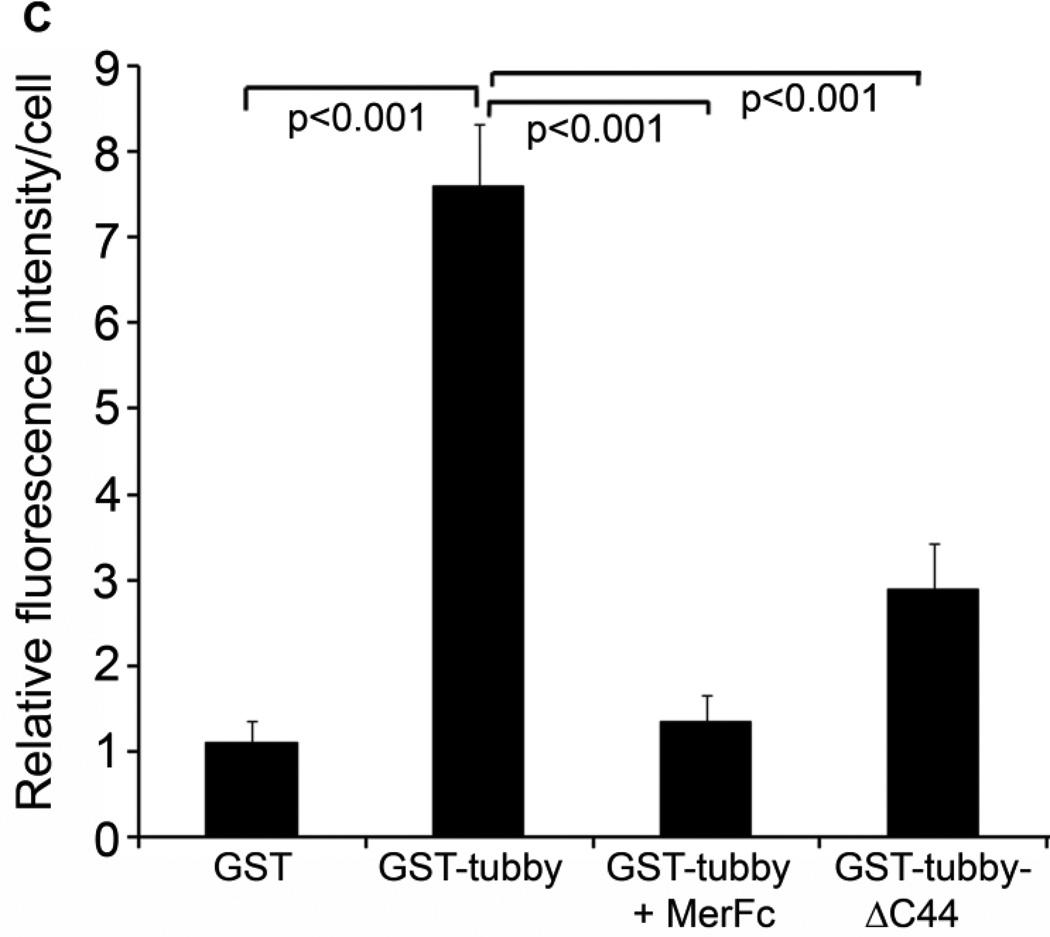
Tubby facilitates microglial phagocytosis through MerTK receptor. (A) Tubby induces MerTK activation in a dose-dependent manner. BV-2 cells were incubated with GST-tubby or GST control at the indicated concentrations. MerTK was precipitated with anti-MerTK antibodies from the cell lysates, analyzed by Western blot using anti-phospho-MerTK or anti-MerTK antibodies. (B) Co-localization of phagocytosed cargos with NMMII-A. pHrodo-labeled plasma membrane vesicles were preincubated with GST-tubby, GST-tubby-ΔC44 or GST (10 nM), washed to remove the unbound proteins and incubated with BV-2 microglial cells for phagocytosis in the presence or absence of excess MerFc (2.5 µg/ml). NMMII-A was detected using anti-NMMII-A antibodies and FITC-labeled secondary antibodies. Co-localization of pHrodo signals and FITC was analyzed by confocal microscopy. Bar = 25 µm. (C) Relative fluorescence intensity of pHrodo per cell in (B) was quantified in more than 100 cells per group (± SEM, n>100; Tukey-Kramer test).
The correlation of tubby concentration curves between microglial phagocytosis and MerTK activation suggests that MerTK is a main pathway for tubby-mediated phagocytosis. An interesting observation is that tubby at a high concentration (200 nM) had a reduced activity to stimulate microglial phagocytosis (Fig. 1B) but minimal decrease in MerTK phosphorylation (Fig. 3A). Similarly, our recent study showed that Gal-3 at a high concentration (1,000 nM) had a reduced activity to stimulate RPE phagocytosis without decrease in receptor phosphorylation (Caberoy et al., 2012). Both tubby and Gal-3 are bridging molecules that link extracellular cargos to MerTK (Caberoy et al., 2012; Caberoy et al., 2010c). A possible explanation for the discrepancy between functional activity and receptor phosphorylation is that bridging molecules at high concentrations may have a reduced efficiency to bridge phagocytosis cargos or preys to phagocytes via the same bridging molecules. An analogy of this phenomenon is the reduced efficiency of plasmid ligation with excess DNA inserts. This observation should be further verified for other bridging molecules with different phagocytic receptors.
3.4. Tubby induces MerTK-dependent NMMII rearrangement
Phagocytosis process requires the rearrangement of cytoskeletal proteins and extension of the plasma membrane for cargo engulfment. MerTK activation is known to induce NMMII rearrangement (Strick et al., 2009). We demonstrated co-localization of NMMII-A with pHrodo signals of internalized cargos (Fig. 3B,C), suggesting that tubby-MerTK interaction induced NMMII rearrangement to facilitate engulfment of apoptotic cells. Excessive MerFc blocked tubby-induced NMMII-A rearrangement as well as the internalization of pHrodo-labeled cargos. Deletion mutation of tubby C-terminal 44 amino acids (Tubby-ΔC44) associates with adult-onset obesity, progressive retinal and cochlear degeneration with undefined mechanisms (Ikeda et al., 2002). The C-terminal domain was mapped as a phagocytosis prey-binding domain (PPBD) that is essential for tubby to bind to apoptotic cells and bridge the phagocytosis preys to phagocytes for engulfment (Caberoy et al., 2010c). Deletion of the PPBD abolished tubby-mediated microglial phagocytosis (Fig. 3B,C).
3.5. Tubby stimulates phagocytosis in primary microglia
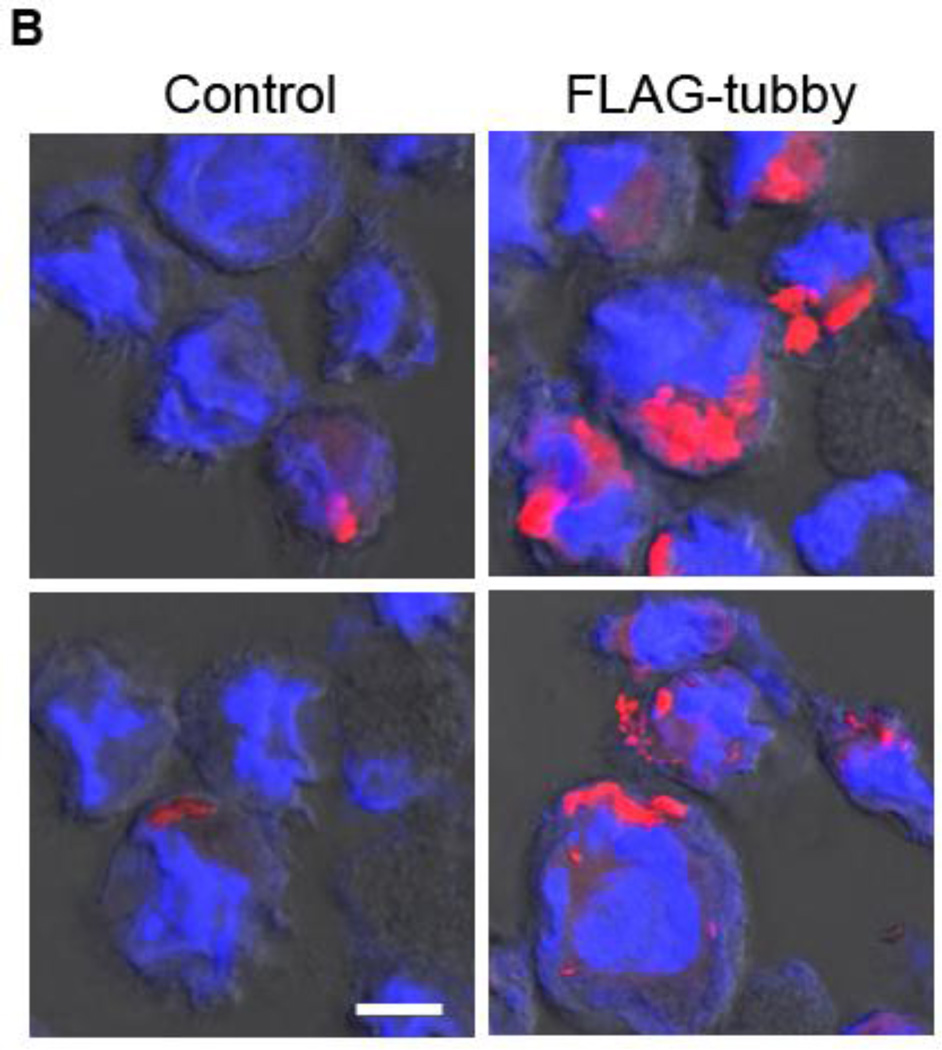
In addition to BV-2 microglial cell line, we characterized tubby-induced engulfment in primary microglia. We prepared primary microglia from neonatal mouse brain and analyzed their phagocytosis in the presence or absence of purified tubby. The results showed that tubby induced phagocytosis of membrane vesicles in primary microglia as well (Fig. 4). The pHrodo signals were clearly inside the primary microglia. These results suggest that BV-2 and primary microglia have similar phagocytosis machinery.
Fig. 4.
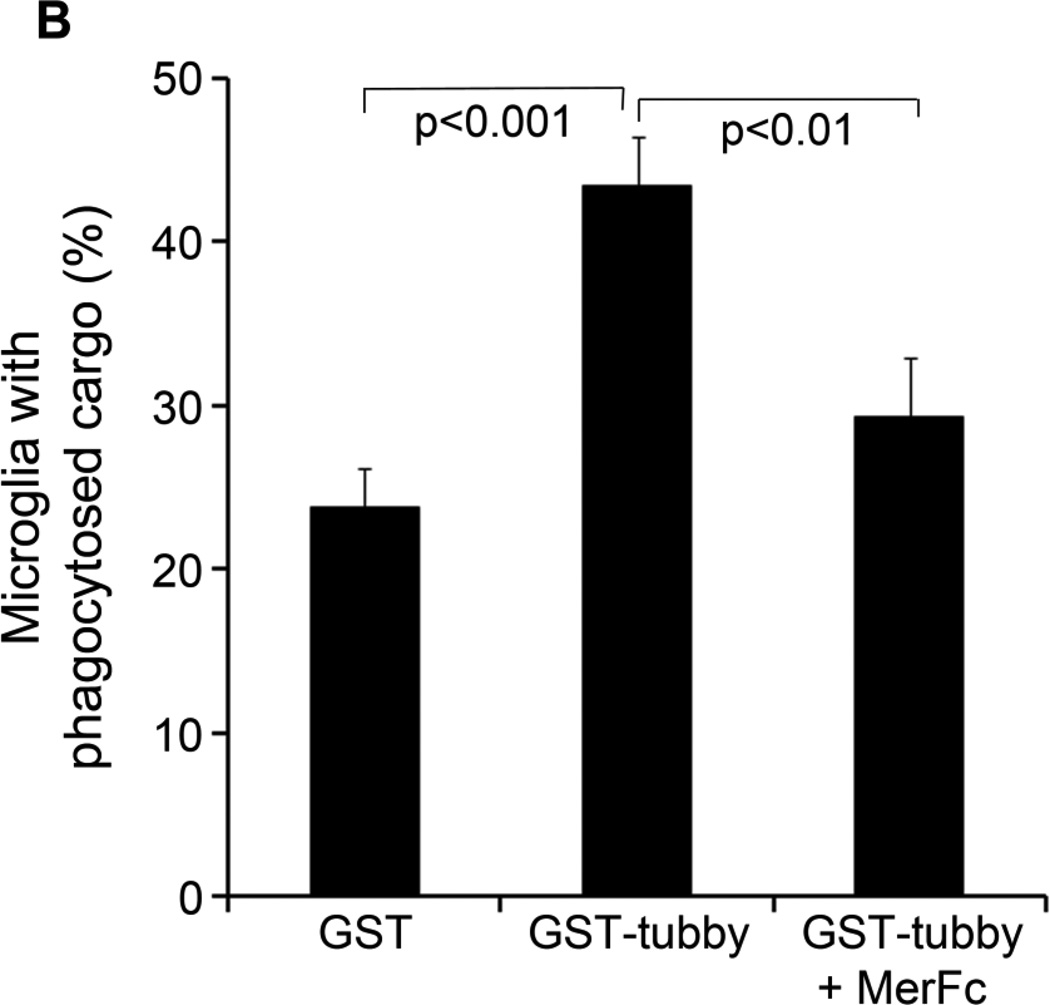
Tubby facilitates primary microglial phagocytosis. (A) Primary microglial phagocytosis. pHrodo-labeled membrane vesicles were preincubated with GST-tubby or GST, washed, incubated with primary neonatal microglia for phagocytosis in the presence or absence of excess MerFc and analyzed by confocal microscopy as in Fig. 3. Bar = 20 µm. (B) Percentage of microglia with internalized pHrodo signal were quantified (± SEM, n>100; t-test).
3.6. Neonatal microglia versus aged microglia
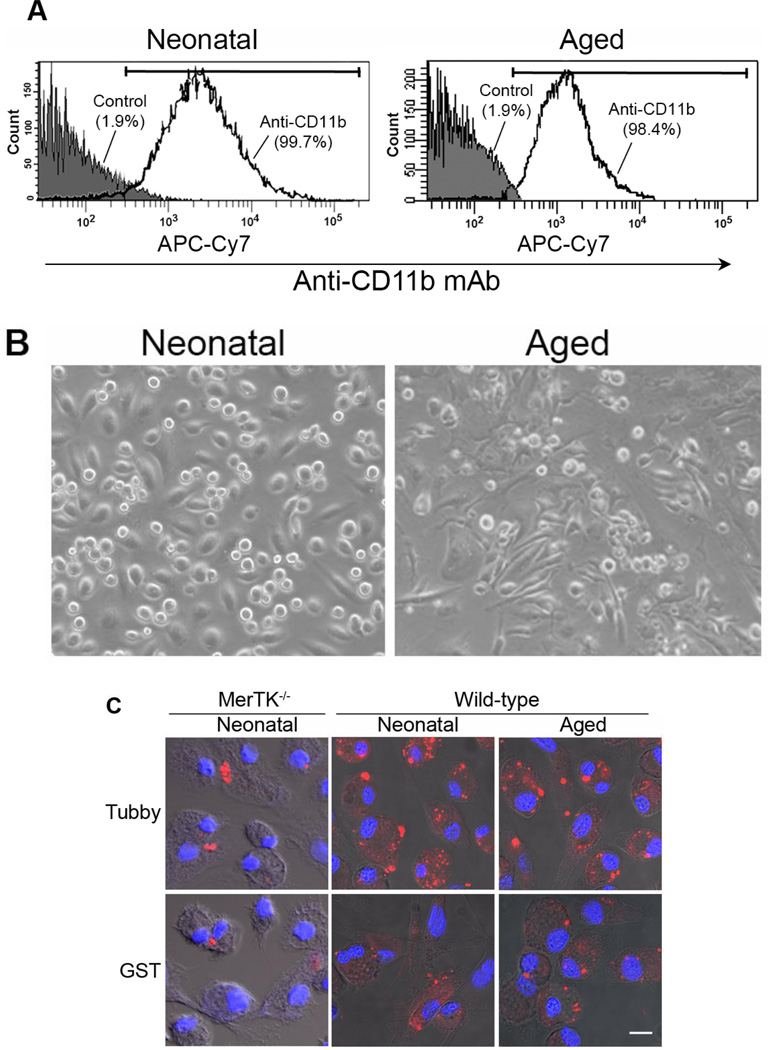
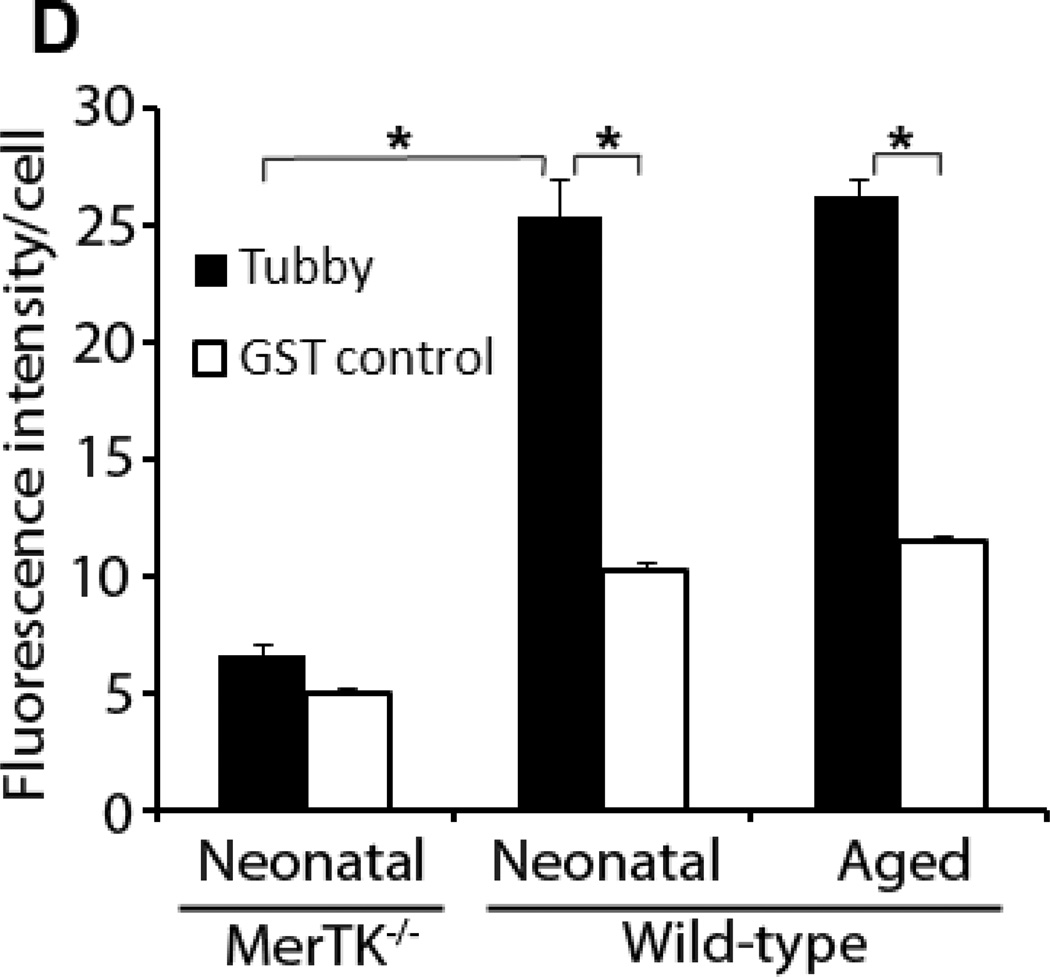
Microglial senescence with increased susceptibility to activation has been implicated in age-related neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease (Lee and Landreth, 2010; Luo et al., 2010; von Bernhardi et al., 2010). One of interesting questions is whether tubby has similar capacity to stimulate phagocytosis in neonatal and aged microglia. We prepared neonatal microglia from mouse brain at postnatal day 4–8 and aged microglia from mice at 15 months of age. Although the yield of aged microglia was much lower than neonatal microglia, both cells had similar purity (Fig. 5A). Aged microglia exhibited more extended cellular processes (Fig. 5B). Neonatal and aged microglia were analyzed for their phagocytosis activity in the presence or absence of tubby. The results showed that neonatal and aged microglia have similar capacity to ingest pHrodo-labeled membrane vesicles in a tubbydependent manner (Fig. 5C,D), suggesting that MerTK-mediated microglial phagocytosis pathways is age-independent. In addition, neonatal microglia with MerTK deletion exhibited reduced phagocytic capacity, suggesting the essential role of MerTK in tubby-mediated microglial phagocytosis.
Fig. 5.
Tubby stimulates phagocytosis by neonatal and aged microglia with similar activities. (A) Purity of primary microglia. Neonatal and aged microglia were prepared from mouse brain at 4–8 days and 15 months of age, respectively, and analyzed by flow cytometry using APC/Cy7-labeled anti-CD11b antibodies. The purity of neonatal and aged microglia was 99.7% and 98.4%, respectively. (B) Morphology of primary microglia under light microscope (20X amplification). (C) Phagocytosis by neonatal or aged microglia. pHrodo-labeled membrane vesicles were prepared and used for microglial phagocytosis as in Fig. 2. Bar = 10 µm. (D) Relative fluorescence intensity of pHrodo per cell in (C) was quantified in more than 100 cells per group (± SEM, n>100, *p<0.001).
4. Discussion
MerTK is a well-characterized phagocytic receptor with immunosuppressive signaling to maintain innate immune balance (Lemke and Rothlin, 2008; Ravichandran and Lorenz, 2007). The role of MerTK is less well defined for the phagocytosis in microglia than RPE and macrophages because mutations of MerTK cause retinal degeneration and autoimmunity, but not neurodegeneration (Gal et al., 2000; Scott et al., 2001). The underlying molecular mechanisms for the tissue-specific degeneration remain an enigma. Tubby is a newly-identified MerTK ligand for RPE phagocytosis and is expressed in embryonic and adult brain as well as the retina (Ikeda et al., 1999; Sahly et al., 1998). This study characterized tubby as a MerTK-specific ligand for microglial phagocytosis, suggesting that tubby-mediated phagocytosis plays an important role in the maintenance of CNS homeostasis.
The beneficial and detrimental roles of microglial phagocytosis have been debated in the past decade in various physiological and pathological conditions. Microglial phagocytosis of foreign pathogens is an important part of the immune defense for neural protection, but activated microglia release pro-inflammatory cytokines and neurotoxic mediators with possible collateral damage on neurons (Rock and Peterson, 2006). While microglial phagocytosis of autoantigens may lead to antigen presentation, autoimmune responses and neuronal damage as in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) (Almolda et al., 2011), phagocytic clearance of autologous apoptotic cells and debris is necessary for resolution of inflammation and neuronal regeneration (Napoli and Neumann, 2010). Immunologically-silent microglial phagocytosis is critical for removal of superfluous neurons during neurogenesis to prevent apoptotic neurons from releasing intracellular contents and triggering inflammation and/or autoimmunity (Erwig and Henson, 2007; Nagata et al., 2010). However, the beneficial role of microglial phagocytosis of apoptotic cells is complicated by possible phagocytosis-mediated killing of stressed but viable neurons during neuroinflammation (Neher et al., 2012; Neher et al., 2011). Blockade of ligand-dependent phagocytosis was shown to preserve live neurons, and deletion of MFG-E8, a phagocytosis ligand for vitronectin receptor, reduced neuronal loss (Fricker et al., 2012). These studies highlight the controversial effects of microglial phagocytosis on neurons. Our results in this study provide a molecular basis for future investigation of tubby-MerTK phagocytosis pathway in neuronal loss during neurogenesis and neuroinflammation.
Molecular phagocyte biology, including phagocytosis ligands, receptors and intracellular signaling cascades, is critical to understanding these controversies (Li, 2012). Of all these signaling molecules, phagocytosis ligands are particularly important because they are the key to selecting extracellular cargos for clearance, initiating the engulfment process, understanding phagocyte functional roles and regulating phagocyte activities with therapeutic potentials (Li, 2012). Like all other cellular ligands, microglial phagocytosis ligands are traditionally identified on a case-by-case basis with technical challenges. As a result, phagocytosis ligands for many other receptors, such as TREM2 and signal regulatory protein-β1 (SIRPβ1), are yet to be identified (Hsieh et al., 2009; Neumann and Takahashi, 2007). We recently developed phagocytosis-based functional cloning to identify unknown phagocytosis ligands in the absence of receptor information (Caberoy et al., 2010a; Li, 2012). Identified Tulp1 and tubby were independently verified to prove the validity of this new approach (Caberoy et al., 2010a; Caberoy et al., 2010c). The results of this study further demonstrated tubby as a biologically-relevant MerTK ligand for microglial phagocytosis, suggesting that our phagocytosis-based functional cloning can be applied to microglia. We further developed dual functional cloning strategy, which combined phagocytosis-based functional cloning with receptor-based affinity cloning, for unbiased identification of receptor-specific ligands (Caberoy et al., 2012; Li, 2012). The identification and characterization of Gal-3 as a new MerTK ligand further supported the validity of our dual functional cloning strategy (Caberoy et al., 2012). Conceivably, these new approaches will improve our capability to identify unknown ligands for TREM2, SIRPβ1 and other receptors to unravel the molecular mystery of microglial phagocytosis.
Microglial senescence contributes to brain aging with increased susceptibility to activation in age-related neurodegenerations, such as Alzheimer’s disease and Parkinson’s disease, and activated microglia likely exacerbate neuroinflammation and neuronal loss (Luo et al., 2010; von Bernhardi et al., 2010). Microglia play an important role in the clearance of amyloid β peptide (Aβ) to minimize the accumulation of amyloid plaque in Alzheimer’s disease (Lee and Landreth, 2010). A recent study showed that aged microglia have reduced capacity to internalize Aβ compared to neonatal microglia (Njie et al., 2010). Although our results indicated that tubby-MerTK pathway has minimal difference between neonatal and aged microglia, characterization of aged-dependent phagocytosis pathways through different receptors will provide molecular insights into microglial functional senescence with increased activation in aged brain. Our phagocytosis-based functional cloning (Li, 2012) will help identify unknown phagocytosis ligands with differential activities in neonatal and aged microglia and facilitate the delineation of microglial functional senescence.
Acknowledgements
This project was supported by NIH R01GM094449 (W.L.), NIH R01EY016211-05S1 (W.L.), K99EY020865 (N.B.C.), P30-EY014801 and an institutional grant from Research to Prevent Blindness. We thank Gabriel Gaidosh for the confocal service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci. 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- Caberoy NB, Alvarado G, Bigcas JL, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227:401–407. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Maiguel D, Kim Y, Li W. Identification of tubby and tubby-like protein 1 as eat-me signals by phage display. Exp Cell Res. 2010a;316:245–257. doi: 10.1016/j.yexcr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Jiang X, Alvarado G, Li W. Efficient identification of tubby-binding proteins by an improved system of T7 phage display. J Mol Recognit. 2010b;23:74–83. doi: 10.1002/jmr.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Li W. Can phage display be used as a tool to functionally identify endogenous eat-me signals in phagocytosis? J Biomol Screen. 2009;14:653–661. doi: 10.1177/1087057109335679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J. 2010c;29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol. 2007;171:2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, Brown GC. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci. 2012;32:2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–140. doi: 10.1007/s11481-007-9090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Nishina PM, Naggert JK. The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci. 2002;115:9–14. doi: 10.1242/jcs.115.1.9. [DOI] [PubMed] [Google Scholar]
- Ikeda S, He W, Ikeda A, Naggert JK, North MA, Nishina PM. Cell-specific expression of tubby gene family members (tub, Tulp1,2, and 3) in the retina. Invest Ophthalmol Vis Sci. 1999;40:2706–2712. [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Eat-me signals: Keys to molecular phagocyte biology and "Appetite" control. J Cell Physiol. 2012;227:1291–1297. doi: 10.1002/jcp.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Current opinion in neurobiology. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Miksa M, Komura H, Wu R, Shah KG, Wang P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 2009;342:71–77. doi: 10.1016/j.jim.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. J Neurosci Methods. 2010;187:243–253. doi: 10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol. 2010;225:24–28. doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Frontiers in pharmacology. 2012;3:27. doi: 10.3389/fphar.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain : a journal of neurology. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Rock RB, Peterson PK. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroimmune Pharmacol. 2006;1:117–126. doi: 10.1007/s11481-006-9012-8. [DOI] [PubMed] [Google Scholar]
- Sahly I, Gogat K, Kobetz A, Marchant D, Menasche M, Castel M, Revah F, Dufier J, Guerre-Millo M, Abitbol MM. Prominent neuronal-specific tub gene expression in cellular targets of tubby mice mutation. Hum Mol Genet. 1998;7:1437–1447. doi: 10.1093/hmg/7.9.1437. [DOI] [PubMed] [Google Scholar]
- Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K, Peri F. Microglia in the developing brain: from immunity to behaviour. Current opinion in neurobiology. 2011;21:5–10. doi: 10.1016/j.conb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Strick DJ, Feng W, Vollrath D. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci. 2009;50:2427–2435. doi: 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]