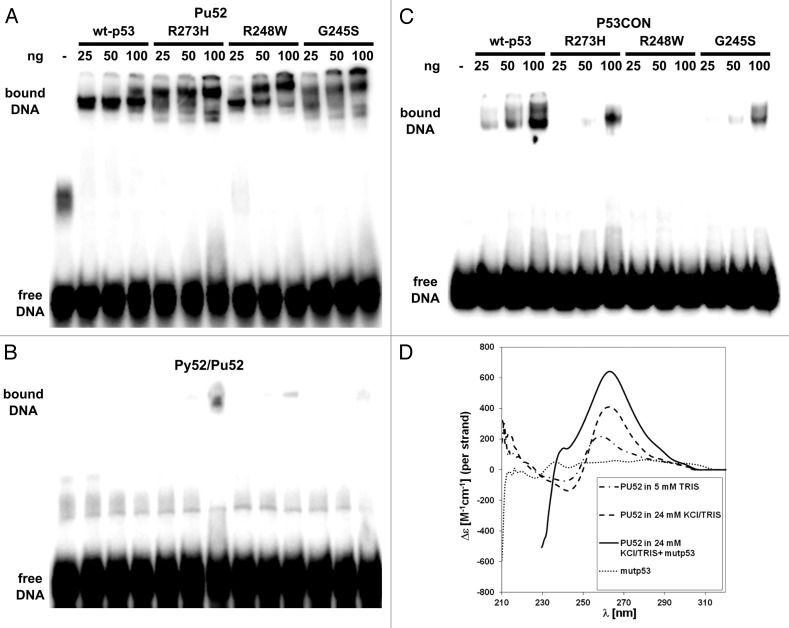
Figure 2. Analysis of the interaction of mutant p53 proteins and of wtp53 with the MYC-PU52 quadruplex structure by EMSA and CD spectroscopy. (A-C) Comparison of the binding affinity of mutant p53 and of wtp53 for the MYC-Pu52 intramolecular quadruplex structure (A), double-stranded (ds) oligonucleotide (Py52/Pu52) (B) and p53CON ds sequence (C). Twenty-five, 50 and 100 ng of wtp53, G245S, R248W and R273H mutant p53 were incubated under binding conditions (see M&M) with 32P-radiolabeled DNA oligonucleotides and 30 ng competitor DNA. (D) CD spectra of the MYC-Pu52 oligonucleotide (0.5 µM) in 5 mM TRIS-HCl, supplemented with 24 mM KCl, supplemented with mutp53R273H (3 µM) and 24 mM KCl. CD spectra were recorded after 24 h when equilibrium was attained. The dotted line corresponds to the CD spectrum of mutp53 alone (0.5 µM). Strong absorption of protein shifts measurement to shorter wavelength.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.