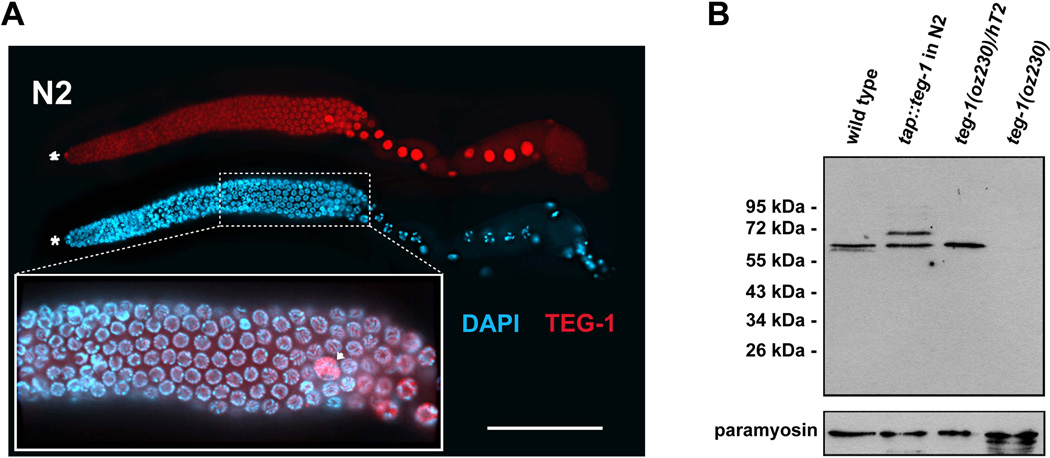
Figure 5.
TEG-1 is nuclear enriched in all gonadal cells. (A) A dissected gonad arm from a wild-type (N2) adult hermaphrodite, one day past the fourth larval stage grown at 20°C, stained with DAPI (blue) to visualize nuclear morphology, and anti-TEG-1 specific antibodies (red). The distal end of the gonad is to the left. Highest levels of TEG-1 enrichment are observed in nuclei of the distal tip cell (asterisk), sheath cells (arrow head) and oocytes (large nuclei in the proximal half of the gonad arm). The inset shows a blow up of a portion of the pachytene region of the gonad arm, with the DAPI and anti-TEG-1 channels merged. The regions of the nuclei occupied by DNA and TEG-1 appear to be mutually exclusive. Intensity comparisons of oocyte and intestine cytoplasm in wild-type and teg-1(oz230) animals demonstrates that some TEG-1 is present in the cytoplasm (oocyte intensity measurements- 1695±428 (n=10) in N2 vs. 456±258 (n=10) in teg-1(oz230)—Intestine intensity measurements- 1504±218 (n=15) in N2 vs. 596±233 (n=17) in teg-1(oz230)). Scale bar = 20 microns. (B) Western blot showing relative size of the TEG-1 protein, and the specificity of the anti-TEG-1 antibodies. A TAP-tag (HA-His8x-TEV-Myc) was fused to the N-terminus of teg-1 genomic DNA to generate an integrated transgenic line. Proteins at 65 kDa (endogenous TEG-1) and at 72 kDa (TAP-TEG-1) are detected in the extract of the transgenic strain. The comparable expression levels between these two proteins indicates that the TAP-TEG-1 is expressed at a level similar to endogenous TEG-1. Approximately 80 adult hermaphorodites from each C. elegans strain were loaded onto each lane of the SDS-PAGE gel. The wild-type and teg-1(oz230) strains are also marked with unc-32(e189). Paramyosin, detected with MH16 antibodies, was used as a loading control.