Abstract
Study Objectives:
Chronic partial sleep loss is associated with obesity and metabolic syndrome in humans. We used rats with lesions in the ventrolateral preoptic area (VLPO), which spontaneously sleep about 30% less than intact rats, as an animal model to study the consequences of chronic partial sleep loss on energy metabolism.
Participants:
Adult male Sprague-Dawley rats (300-365 g).
Interventions:
We ablated the VLPO in rats using orexin-B-saporin and instrumented them with electrodes for sleep recordings. We monitored their food intake and body weight for the next 60 days and assessed their sleep-wake by 24-h EEG/EMG recordings on day 20 and day 50 post-surgery. On day 60, after blood samples were collected for metabolic profiling, the animals were euthanized and the brains were harvested for histological confirmation of the lesion site.
Measurements and Results:
VLPO-lesioned animals slept up to 40% less than sham-lesioned rats. However, they showed slower weight gain than sham-lesioned controls, despite having normal food intake. An increase in plasma ghrelin and a decrease in leptin levels were observed, whereas plasma insulin levels remained unaffected. As expected from leaner animals, plasma levels of glucose, cholesterol, triglycerides, and C-reactive protein were reduced in VLPO-lesioned animals.
Conclusions:
Chronic partial sleep loss did not lead to obesity or metabolic syndrome in rats. This finding raises the question whether adverse metabolic outcomes associated with chronic partial sleep loss in humans may be due to factors other than short sleep, such as circadian disruption, inactivity, or diet during the additional waking hours.
Citation:
Vetrivelan R; Fuller PM; Yokota S; Lu J; Saper CB. Metabolic effects of chronic sleep restriction in rats. SLEEP 2012;35(11):1511-1520.
Keywords: Obesity, metabolic syndrome, ventrolateral preoptic area, insomnia, sleep restriction
INTRODUCTION
Chronic partial sleep loss due to voluntary sleep restriction has become common in modern day society.1 Chronic partial sleep loss, either voluntary or imposed, has been shown to be associated with adverse effects on daytime performance, cognitive-related tasks, and other functions in humans.2 More recently, chronic partial sleep loss in humans has been linked to obesity and the metabolic syndrome, a clinical condition characterized by increased visceral fat deposition, elevated plasma glucose and triglyceride (TG) levels, insulin resistance, type 2 diabetes mellitus (T2D), and an elevated risk for cardiovascular disease.1,3,4 For example, subjects who habitually sleep ≤ 6 h per day have an increased risk of obesity and have a higher mean body mass index than those sleeping ≥ 9 h per day.5 Van Cauter and colleagues showed that healthy young human volunteers who were restricted to 4 h sleep per night for 6 consecutive nights showed elevated glucose levels and decreased insulin sensitivity.6 Similar sleep restriction but spanning only 2 consecutive nights altered the levels of appetite-regulating hormones leptin and ghrelin, which in turn can increase appetite and may ultimately lead to weight gain.7 Elevated TG and cholesterol (LDL) levels have also been documented in sleep-restricted humans.8 In addition, the metabolic syndrome is considered a pro-inflammatory state, and sleep loss produces increases in the plasma levels of inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) in humans.9–11 Collectively, these results indicate that chronic sleep loss could potentially increase the risk for obesity and T2D in humans. However, all long-term studies performed thus far have been epidemiological and so provide only a correlative linkage between short sleep periods and obesity. The short-term studies on healthy volunteers, by contrast, have not provided insight into the long-term effects of sleep restriction on metabolic function.
Because it is difficult to control diet and sleep behavior in humans over long periods of time, studies in animal models are ultimately critical to understanding whether sleep loss is causally linked to obesity and T2D development. Moreover, animal models will be necessary for delineating the underlying neural mechanisms linking sleep loss with metabolic dysregulation. Animal studies in the current literature have employed acute or chronic total sleep deprivation protocols, which are not comparable to humans who experience sleep loss for a few hours per day on a chronic basis, rather than total sleep deprivation. In addition, the sleep deprivation procedures employed in animal models have necessarily included the introduction of external stressors, ranging from handling by humans to forced locomotion or threat of water immersion if the animal falls asleep. Both stress and excessive movement alter feeding and energy metabolism, so the independent effect of sleep loss is not clear.12 Moreover, these sleep deprivation studies generally affect the circadian patterns of other activities including feeding, and such circadian dysregulation has been shown to influence metabolic regulation.13,14 We would therefore argue that, to date, there has not been an adequate method for enforcing chronic partial sleep deprivation in rodents without the introduction of a chronic stress confound or without affecting the circadian pattern of other activities.
In the present study, we used rats with bilateral lesions of the ventrolateral preoptic nucleus (VLPO) as an animal model to investigate the effects of chronic partial sleep loss on energy metabolism. Studies from our lab and others have established that the VLPO in the hypothalamus is a critical structure for the regulation of sleep.15–20 Rats with lesions of the VLPO (VLPOx) lose up to 50% of their daily sleep, and this sleep loss continues for at least 3 weeks following lesions with no sign of recovery.17 This makes VLPOx animals a useful model for studying the effects of chronic partial sleep loss without continual stressful experimental interventions. Also, and importantly, VLPO lesions have minimal impact on the circadian rhythms of sleep, locomotion, or body temperature in rats17; thus the confounding impact of circadian dysregulation is avoided. In the present study, we employed the VLPOx rat model to investigate the effects of chronic partial sleep deprivation for 2 months on metabolic regulation.
MATERIALS AND METHODS
Animals
Pathogen-free adult male Sprague-Dawley rats (n = 41, 300-365 g, Harlan) were used for the study. The rats were housed in individual cages. The cages were housed inside isolation chambers, which provided ventilation, computer-controlled lighting (12:12 light-dark cycle, lights on at 07:00; 200 lux), an ambient temperature of 22 ± 1°C, and visual isolation. Care of the rats met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the BIDMC and Harvard Medical School Institutional Animal Care and Use Committees.
Surgery
Under anesthesia (chloral hydrate, 350 mg/kg body weight), the rats were placed in a stereotaxic apparatus. A fine glass pipette containing orexin-B-saporin (OX-SAP; Advanced Targeting Systems, CA, USA) solution was lowered to coordinates (AP −0.6 from bregma, L ± 1.00, DV 8.5) corresponding to the VLPO as per the rat atlas of Paxinos and Watson.21 In 34 animals, OX-SAP (200 nL of 0.1% solution) was injected using a compressed air delivery system as described previously.22,23 One group of animals (n = 7) received 200 nL saline into the VLPO and served as sham lesioned (sham-L) controls. Following the injections, 4 EEG screw electrodes were implanted into the skull, in the frontal (2) and parietal bones (2) of each side, and 2 flexible EMG wire electrodes were placed into the neck muscles for the collection of sleep-wake data. The free ends of the leads were soldered into a socket that was attached to the skull with dental cement, and the incision was then closed by wound clips.17,24
Food Intake and Body Weight Measurements
Following the surgical procedure, the rats were maintained for 60 days, during which the food intake of the rats was measured daily (barring the days of habituation and recording) and body weight was measured once a week. Rats were fed with regular rat chow (Cat. No 5008; Formulab, USA) containing 26.8% protein, 16.7% fat, and 56.4% carbohydrate during the entire period of study. Body weight gain during the 60-day experimental period was calculated by subtracting the initial body weight on day 0 (the day of surgery) from the body weight on week 8 (the day of perfusion).
Sleep-Wake (S-W) Recording and Analysis
S-W recordings were carried out on day 20 and on day 50 post-lesion from each animal. The rats were connected via flexible recording cables to a commutator, which in turn was connected to a Grass polygraph and computer. The rats were habituated to the cables for 2 days. Recordings of the EEG/EMG and time-lock video began after the habituation period and continued uninterrupted for 24 h on each occasion. The digitized EEG/EMG data of each rat was divided into 12-sec epochs and visually scored as wake, non rapid eye movement sleep (NREM) sleep, or rapid eye movement (REM) sleep as described previously.17 Scoring was done before histological examination, so the scorer was unaware of the extent of the lesions. The daily percentage of time spent in wake, NREM sleep, and REM sleep and frequency and durations of episodes of each stage were calculated. Finally, the percentage of sleep loss in each VLPOx rat was calculated as 100 – (percentage of total sleep (NREM + REM) in each VLPOx rat × 100 / mean percentage of total sleep in sham-L rats).
Histology
On completion of the recordings, the rats were fasted overnight (∼15 h), then killed (between 10:00 and 11:00 to avoid circadian variation in the results) by deep anesthesia with 500 mg/kg chloral hydrate. The chest was opened and blood (5 mL) was withdrawn from the right atrium of the heart (see below), following which the animals were perfused through the heart with saline (100 mL), followed by 500 mL of neutral phosphate buffered formalin (Fischer Scientific Co.). The brains were harvested, post-fixed, and sectioned in the coronal plane on a freezing microtome into 4 series of 40 μm sections. One series was processed for Nissl staining as described previously.17
Lesion sites were identified based on neuronal loss and gliosis, and the lesion size was then quantified by counting the remaining number of neurons in the VLPO. Cell counts were made bilaterally on 3 sections (one every 160 μm) for the VLPO cluster, medial extended VLPO, and dorsal extended VLPO region. The construction of counting boxes for these structures (see Figure 1) was similar to that explained in our previous publication.24 Only cells with a clear nucleus were counted, nuclear diameters were quantified for a sample of 20 neurons in each field in each animal, and cell counts were corrected using Abercrombie's formula.25 Percentage of cell loss in each of these VLPO subregions was estimated as 100 – (remaining number of neurons in that subregion of the VLPO in each VLPOx rat × 100 / mean cell count in the same region in sham-L rats). The approximate extent of the lesions in each case was plotted on templates for the purpose of comparison (Figure 1).
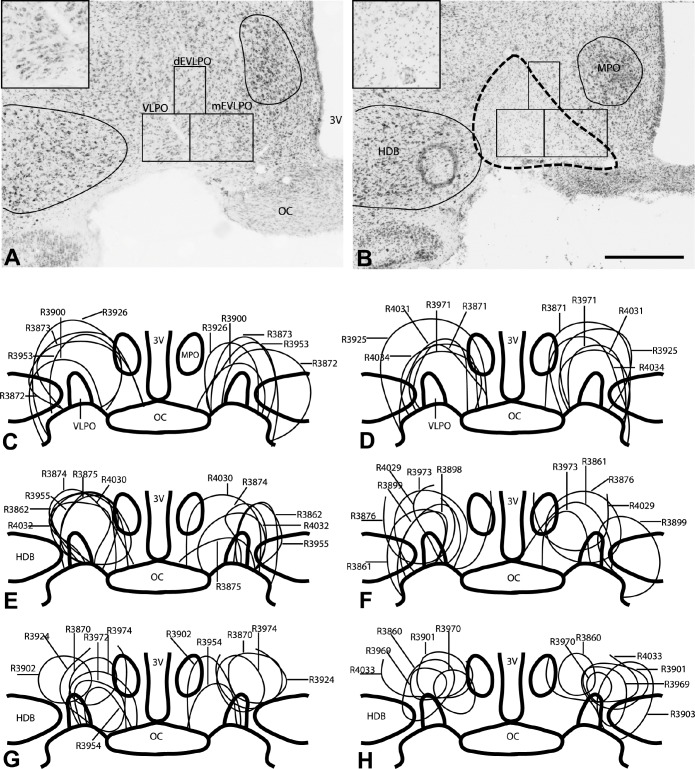
Figure 1.
Nissl stained brain sections from a rat that received saline into the VLPO (A) and a rat that received orexin-B-saporin into the VLPO (B). The outline of the lesion, marked by loss of large, darkly stained neurons and excess small glial nuclei, is shown in panel B (dashed line). This is better seen in enlarged views of the VLPO cluster from these sections in the corresponding insets. Counting boxes were placed over the VLPO cluster (VLPO), dorsal extended VLPO (dEVLPO), and medial extended VLPO (mEVLPO) as described in our previous study,24 and the number of remaining neurons were counted and then correlated with sleep and metabolic outcomes. Panels C-H show schematic representations of the location and extent of the lesions in each animal that received OX-SAP (n = 34). Note that a few animals had only unilateral lesions and are illustrated on only one side of the brain (R3898 in panel F and R3903 and R3860 in panel H). 3V, third ventricle; OC, optic chiasm; VLPO, ventrolateral preoptic area cluster; dEVLPO, dorsal extended VLPO; mEVLPO, medial extended VLPO; HDB, diagonal band of Broca horizontal; MPO, medial preoptic area. Scale bar = 500 μm.
Blood Collection and Analysis
During the perfusion procedure, approximately 5 mL of blood was obtained from the right atrium of each animal and stored in tubes containing heparin. Blood samples were then centrifuged at 5,700 rpm for 5 min at 4°C. Plasma was collected in polycarbonate tubes and frozen for further determination of plasma levels of various metabolic parameters: glucose, insulin, cholesterol, TG, adiponectin, ghrelin, leptin, and CRP. All these assays were performed at the Specialized Assay Core Facility, Joslin Diabetes Center, Boston, USA using commercially available kits. ELISA kits for insulin (Cat. No. 90060; Crystal Chem Inc, IL, USA; sensitivity 5 pg/mL), leptin (Cat. No. 90040; Crystal Chem Inc, IL, USA; sensitivity 200 pg/mL), adiponectin (Cat. No. EZRADP-62K; Millipore, MA, USA; sensitivity 0.155 ng/mL), ghrelin (Cat. No. 32-5119; ALPCO Diagnostic, NH, USA; sensitivity 0.6 pg/mL) and CRP (Cat. No. 557825; BD, NJ, USA; sensitivity 4.2 ng/mL) were used for their respective assays. Glucose, TG, and cholesterol were determined by enzymatic and colorimetric methods (Glucose kit - Cat No; 439-90901; sensitivity linear to 700 mg/dL; Wako Diagnostics; TG kit - Cat No; 2100-430; sensitivity linear to 1,000 mg/dL; Stanbio Laboratory, TX and cholesterol kit -Cat No; 1010-430; sensitivity linear to 750 mg/dL; Stanbio Laboratory, TX, USA respectively). We also attempted to assay tumor necrosis factor α (TNF-α), but the plasma values in all but a few animals were below the limits of detection of the assay (TNF-α kit: Cat. No. 45-TNFRT-E01.1; ALPCO Diagnostics, NH, USA; sensitivity 4 pg/mL).
Statistical Analysis
To analyze the effects of successful VLPO lesions on sleep-wake parameters including total time spent in each stage, their mean bout durations, and bout numbers, we used unpaired t-tests to compare the sham-L rats with animals who sustained > 70% cell loss in the VLPO cluster, a threshold we had determined in our previous work as causing profound sleep loss.17 For the analysis of metabolic parameters including food intake, body weight, and plasma levels of various metabolic factors, we used unpaired t-tests to compare the sham-L animals to the same group of VLPOx animals with > 70% cell loss, but also did a second analysis comparing the sham-L animals with a larger group of rats with > 10% sleep loss, which represented a threshold similar to 1 h/day sleep loss in humans, a range often seen in the general population. For the correlation of cell loss in the VLPO cluster with the daily percentage of wake, we analyzed the entire group of VLPOx animals. Similarly, for correlation analysis of metabolic variables, the amount of each variable was plotted against daily percentage of wake, percentage of cell loss in the VLPO cluster, and then with the cell loss in the entire VLPO region (including the VLPO cluster and medial and dorsal extended VLPO). Pearson correlation coefficients and P values were calculated using SPSS v17 (SPSS Inc, Chicago, USA).
RESULTS
OX-SAP-Induced VLPO Lesions
In our cohort of 34 animals that received OX-SAP, cell loss in the VLPO cluster ranged from about 1% to 100%, depending upon the injection location and spread (see Figure 1). Although the injections were targeted at the VLPO cluster, most of them extended into the medial and dorsal extended VLPO. Despite extending medially into the ventrolateral part of the medial preoptic nucleus (see Figure 1B), none of the lesions extended sufficiently medially to involve the midline median preoptic nucleus (MnPO). Some lesions extended laterally to a small extent into adjacent basal forebrain areas—the nucleus of the diagonal band of Broca (DBB) or the substantia innominata (SI). Fortunately, lesions of this size in the basal forebrain region have minimal effects on sleep-wake.26,27 Hence, we performed cell counts in the VLPO cluster and the medial and dorsal extended VLPO regions (Table 1) to correlate with the observed sleep-wake behavior in these animals (Figure 2).
Table 1.
The remaining number of neurons in the ventrolateral preoptic (VLPO) subregions after saline (sham-L rats) or OX-SAP injections into the VLPO (VLPOx rats)
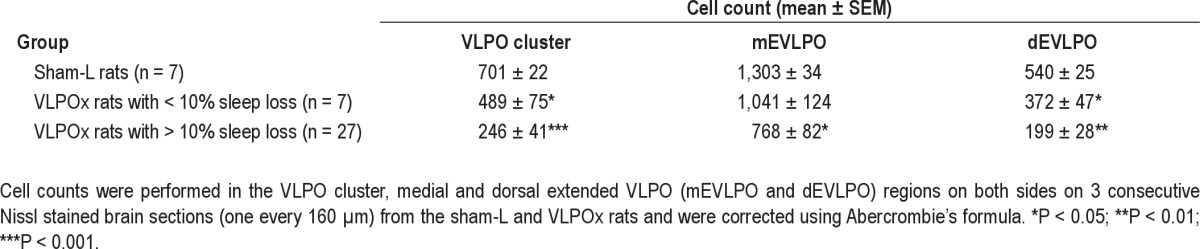
Figure 2.
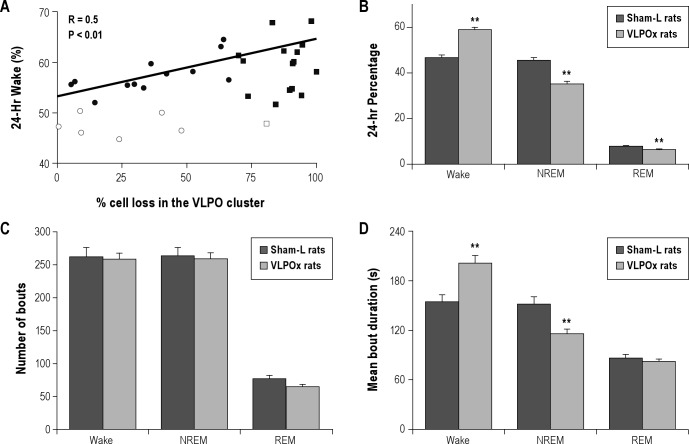
Changes in sleep-wakefulness following lesions in the ventrolateral preoptic area (VLPO). Sleep-wake recordings were carried out on day 20 and on day 50 post-lesion from each animal and for 24 h on each occasion. As there were no significant differences in sleep-wake parameters between these 2 recordings, the mean values were calculated for each animal and were used for further analysis. (A) Daily percentage of wake in the entire cohort of animals where we attempted VLPO lesions was positively correlated with the percentage of bilateral cell loss in the VLPO. These animals were then classified in 2 ways—based on their cell loss in the VLPO cluster (> or < 70% cell loss) and based on sleep loss they experienced (> or < 10% sleep loss). Filled circles (•) = rats with < 70% cell loss in the VLPO but displayed > 10% sleep loss (n = 12). Open circles (∘) = rats with < 70% cell loss in the VLPO and had < 10% sleep loss (n = 6). Filled squares (■) = rats with > 70% cell loss and displayed > 10% sleep loss (n = 15). Open square (▫) = rat with > 70% cell loss in the VLPO but had < 10% sleep loss (n = 1). (B) Daily percentages of sleep-wake stages in sham-lesioned (sham-L; dark bars) animals (n = 7) and VLPO-lesioned (VLPOx; light bars) animals with > 70% cell loss (n = 16). VLPO lesions produced significant increase in wakefulness and a corresponding decrease in non-rapid eye movement sleep (NREM) and REM sleep. This sleep loss was brought about through an increase in average duration of wake bouts and a decrease in average duration of NREM sleep (D). The total number of wake, NREM or REM bouts during 24 h did not show any significant difference (C). Values are mean ± SEM **P < 0.01.
Sleep-Wake Changes following VLPO Lesions
S-W data recorded on post-lesion days 20 and 50 showed a marked increase in wakefulness in the group of animals with lesions including the VLPO. As there was no significant difference in the percentages of W (57.02 ± 1.25% on day 20 vs. 55.43 ± 1.03% on day 50), NREM (36.43 ± 1.07% on day 20 vs. 37.96 ± 0.88% on day 50), and REM (6.55 ± 0.25% on day 20 vs. 6.61 ± 0.22% on day 50) between the recordings on post-lesion day 20 and day 50 (P > 0.05; paired t-test) from the entire cohort of animals in which we attempted VLPO lesions (n = 34), the mean values of these 2 periods (day 20 and day 50) were calculated for each animal and used as their daily percentage of sleep-wake stages. As expected, VLPO lesions of varying extent and location produced different degrees of sleep loss, and for this entire cohort, the amount of wake time correlated with the loss of neurons in the VLPO cluster (r = 0.50, P < 0.01; see Figure 2) and with the loss of neurons in the entire VLPO region (r = 0.53, P < 0.01).
Because our earlier study17 found that substantial sleep loss was seen most reliably in rats with > 70% cell loss bilaterally in the VLPO cluster, we then compared the sleep-wake parameters from the animals with > 70% cell loss in the VLPO cluster with those of sham-L animals. On average, animals (n = 16) with > 70% cell loss in the VLPO cluster spent about 26% more time in wakefulness (58.71 ± 1.43%) than sham-L rats (46.57 ± 1.04%) with corresponding decreases in NREM and REM sleep (Figure 2). These animals lost 13.5% of sleep in the light phase (61.8 ± 1.2% vs. 71.5 ± 2.1% in sham-L animals; P < 0.01) and 44.1% of sleep in the dark phase (19.6 ± 1.5% vs. 35.1 ± 2.7% in sham-L animals; P < 0.001). Analysis of the bout numbers and average durations of sleep-wake stages showed that VLPO lesions (> 70% cell loss) caused a significant reduction in NREM bout duration (115.7 ± 4.3 s vs. 151.08 ± 8.6 s in sham-L animals; P < 0.01) and a significant increase in wake bout duration (200.74 ± 8.78 s vs. 154.07 ± 8.08 s in sham-L animals; P < 0.01). However, the total number of daily wake and NREM bouts in these rats with > 70% cell loss in the VLPO cluster was comparable to those of sham-L rats (P > 0.05). In addition, there was also no significant difference in the average duration or number of REM bouts (Figure 2). Furthermore, their diurnal phase and period of the sleep-wake cycle remained unaltered (Figure 3), and the proportion of waking time spent in the light vs. the dark period did not differ significantly from the control animals. For example, about 66.78 ± 1.7% and 67.29 ± 0.8% of total wakefulness was observed during the dark period in sham-L rats and VLPOx rats with > 70% cell loss, respectively (P > 0.05).
Figure 3.
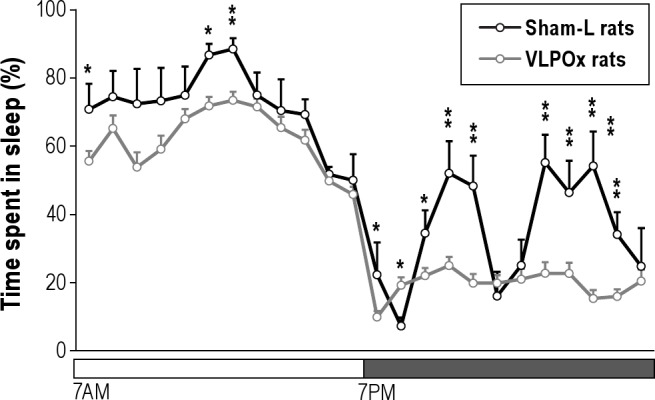
Average hourly percentage of sleep (NREM + REM) for 24 h in sham-lesioned (sham-L) rats (n = 7) and VLPO-lesioned (VLPOx) rats with > 70% cell loss (n = 16), indicating that the overall diurnal pattern of sleep-wake was not affected by VLPO lesions. Values are mean ± SEM; *P < 0.05; **P < 0.01; repeated measures of ANOVA followed by unpaired t-test.
Changes in Food Intake and Body Weight in VLPOx Animals
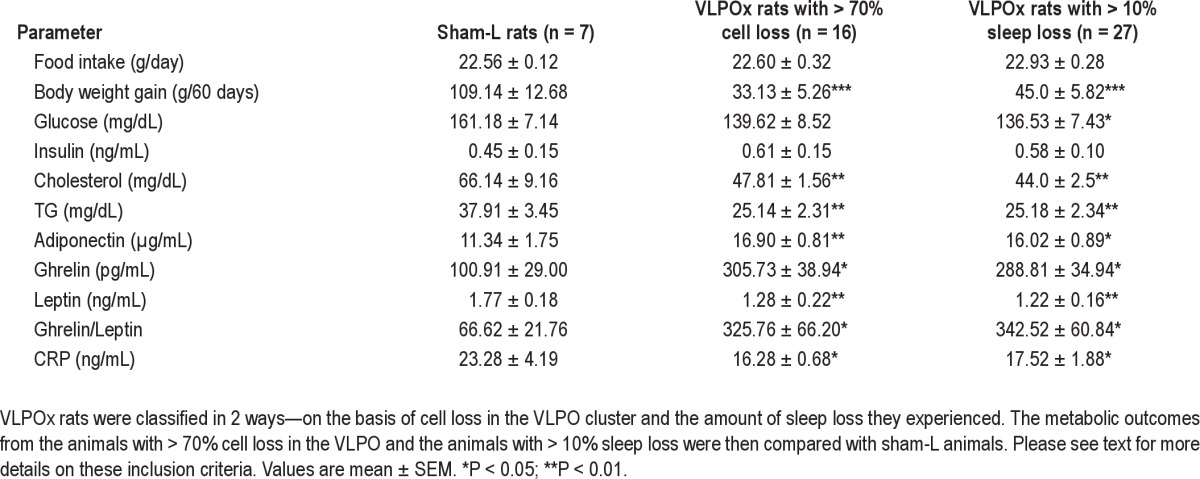
Following sleep-wake analysis from the VLPOx animals, food intake, body weight gain, and other plasma profiles of metabolic parameters from the animals with > 70% cell loss in the VLPO cluster were compared with those from sham-L animals. These results are presented in Table 2. As this cohort of VLPOx animals had about 25% sleep loss, we did a second analysis of all animals with 10% or greater sleep loss (VLPOx-10S; n = 27) to better approximate the human population, in which increased body mass index is seen when sleep is reduced by as little as one hour per day. The metabolic parameters of the entire group of VLPOx-10S animals were compared with those from sham-L animals. Finally, the data from entire cohort of animals with attempted VLPO lesions (shown in Figure 1; n = 34) were used for correlation analysis of metabolic variables with percentage of sleep loss. The results were similar in the animals with > 70% VLPO lesions and the larger group of VLPOx-10S rats (Table 2), hence we will describe them in detail only for the latter comparison, i.e., all animals with ≥ 10% sleep loss.
Table 2.
Plasma profiles of various metabolic markers in the sham-lesioned (sham-L) rats and VLPO-lesioned (VLPOx) rats
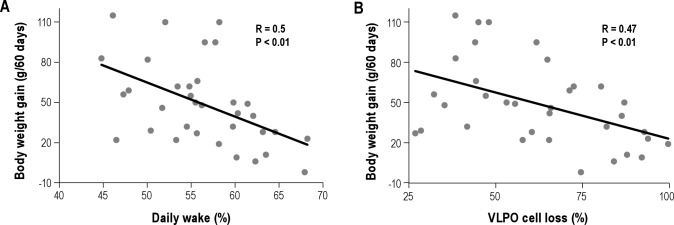
A reduction in food intake was observed in both sham-L (17.49 ± 1.01 g/day) and VLPOx-10S rats (19.88 ± 1.04 g/day) during the first week after surgery (Tukey multiple comparison test, P < 0.001), which is a normal post-surgical response. On the other hand, neither the food intake within the groups (Tukey multiple comparison test, P > 0.05), or between the groups (VLPOx-10S animals; 22.93 ± 0.28 g/day vs. sham-L animals; 22.56 ± 0.12 g/day) differed significantly during the subsequent 7 weeks. Furthermore, the food intake was also not correlated with the percentage of cell loss in the entire VLPO region (R = 0.12; P > 0.05; Pearson correlation) or with the percentage of cell loss in the VLPO cluster (R = 0.04; P > 0.05; Pearson correlation). However, weight gain by VLPOx-10S rats was 59% less than that of the sham-L animals. While the body weight of the VLPOx-10S animals (337.00 ± 3.15 g) was comparable to that of sham-L animals (326.71 ± 7.26 g) on day 0 (P > 0.05), it was significantly lower on day 60 (382.00 ± 5.15 g vs. 435.86 ± 6.62 g in sham-L animals; P < 0.001). Overall, the VLPOx-10S animals gained only 45.0 ± 5.82 g when compared to the sham-L animals that gained 109.14 ± 12.68 g in 60 days (P < 0.001). Similarly, the weight gain in the entire cohort of animals with attempted VLPO lesions was negatively correlated with the percentage of sleep loss (r = 0. 5; P < 0.01; Pearson correlation) and with cell loss in the entire VLPO region (Figure 4; r = 0.47; P < 0.01; Pearson correlation; Figure 4), although not significantly correlated with percentage of cell loss in the VLPO cluster (R = 0.29; P > 0.05).
Figure 4.
Animals with VLPO lesions displayed decelerated weight gain. Across the entire cohort of animals with lesions in the VLPO region (n = 34), the weight gain during the 60-day experimental period was negatively correlated with the daily percentage of wake (A) and the percentage of cell loss in the entire VLPO region (B).
Changes in Plasma Metabolic Profile in VLPOx Animals
Plasma levels of several established metabolic markers from sham-L, > 70% VLPO lesioned, and VLPOx-10S rats are provided in Table 2. Again, the results of the 2 VLPOx groups were very similar, and will be described in detail only for the larger one that includes all animals with ≥ 10% sleep loss.
Although no change in food intake was observed, VLPOx-10S rats exhibited an increase (186 ± 34.63%) in plasma levels of the orexigenic hormone ghrelin and a significant reduction (31 ± 8.92%) in the plasma levels of the anorectic hormone leptin when compared with sham-L animals (Table 2). Thus, the ghrelin/leptin ratio showed a 414.1 ± 91.3% increase in the VLPOx-10S animals. This ratio is often correlated with increased appetite, but in this experiment the VLPOx-10S animals did not eat more than the sham-L animals, although food was available ad libitum, and the animals had a longer opportunity to eat because they spent more of their time awake. Consistent with the lower body mass observed, VLPOx-10S animals displayed significant decreases in plasma levels of glucose (15.3 ± 4.61%), cholesterol (33.52 ± 3.72%), and TG (33.57 ± 6.17%) as compared to sham-L animals. On the other hand, plasma adiponectin levels were elevated by 41.4 ± 7.8% in VLPOx-10S animals, whereas their insulin levels remained unaltered (Table 2). Finally, plasma levels of an inflammatory marker CRP were reduced by 24.75 ± 8.06% in VLPOx-10S animals (Table 2).
DISCUSSION
We evaluated metabolic changes following chronic partial sleep loss using VLPOx rats as our model system. VLPO lesions in rats reduced the amount of sleep by up to 40%, equivalent to reducing sleep from 8 h to 4.8 h per night in humans. Interestingly and in marked contrast to published findings from humans in short term studies, chronic partial sleep loss in VLPOx rats did not result in obesity or the metabolic syndrome. In fact, plasma levels of several key metabolic markers in these animals indicated what is widely considered a “healthier” profile.
VLPOx Animals as a Model for Chronic Partial Sleep Loss
We used rats with bilateral VLPO lesions as an animal model to study the effects of chronic partial sleep loss. As expected, VLPO lesions by OX-SAP produced significant sleep loss in rats that persisted through the final recordings conducted on post-surgical day 50. Hence, and consistent with previous reports,17 sleep loss in VLPOx animals is persistent with little to no compensation occurring over the 2-month experimental period. VLPOx animals therefore provide a stable model system for studying the effects of chronic partial sleep loss. In particular, this model has important advantages over procedures that cause sleep loss by externally applied stressors, such as subjecting the animals to the threat of water immersion or requiring them to move if they fall asleep. Moreover, the amount of sleep loss in VLPOx animals is quantitatively comparable to chronic sleep loss in humans replicating a range of sleep loss from 10% (about 1 h/night) to 40% (equivalent to 4-5 h/night), which is similar to humans who voluntarily curtail nightly sleep in favor of other activities.28
One potential complication with the VLPOx model is that it does involve a brain lesion, which could potentially alter feeding or metabolic responses, independent of the sleep loss. However, this is unlikely because there was no acute or chronic change in feeding in the animals with VLPO lesions in the present study. Over the course of the experiment, the VLPOx animals consistently consumed the same amount of food as sham-L rats. On the other hand, VLPOx animals were awake for more hours each day, and appeared (based upon EEG/EMG recordings) to have longer active periods, which may account for the reduced rate of body weight gain during the 2-month observation period.
The VLPO lesions used in this study differed in several important ways from the ones used by Lu and colleagues.17 Firstly, in this study OX-SAP was used to generate the VLPO lesions whereas ibotenic acid was employed in the previous study. At the dosage used in this study, OX-SAP is not selective,27 and it nonspecifically kills all cell types in the preoptic area. We preferred OX-SAP to ibotenic acid because it acts slowly and hence does not produce unwanted side effects such as excitatory autonomic responses that can compromise circulation or breathing due to transient neuronal activation by ibotenic acid. Secondly, in the previous study the lesions were placed slightly more medially, to avoid including the laterally adjacent basal forebrain (BF), as it was not known at the time whether damage to BF neurons might independently affect sleep amounts. Recent studies have shown that ibotenic acid or OX-SAP injections in the BF that kill fewer than 40% of BF neurons actually increase the amount of delta power in the EEG without causing long-term changes in the amount of sleep.26,27 Hence, to improve the frequency with which our lesions included the entire VLPO we used slightly more lateral injection coordinates, which allowed some injections to include small portions of the adjacent BF, but avoided the median preoptic nucleus (which contains sleep-active neurons, but where lesions may cause reduced drinking and impair electrolyte balance or thermoregulation).29 We attribute the small differences in the sleep physiology following VLPO lesions between this study and our previous study to the lesions systematically including more basal forebrain tissue but less of the medial extended VLPO than in our previous study.17 For instance, while the animals in the study by Lu et al.17 with greater than 80% VLPO cell loss showed up to 55% sleep loss, in this study we did not observe more than 40% sleep loss. Also, in the previous study increased numbers of both wake and sleep bouts were observed after VLPO lesions, whereas in the current cohort, sleep bout duration was shorter, but the numbers of bouts were unchanged.
The unchanged number of sleep bouts in the VLPOx rats further indicates that they have a normal level of sleep drive.30 This was expected because our VLPO lesions were carefully designed to spare the MnPO, which contains neurons that are activated by sleep need or sleep drive, as measured by attempts to enter into sleep.30 The VLPO neurons, on the other hand, are only activated during sleep, suggesting that they convert sleep drive to actual sleep, but do not accumulate sleep need or drive.15,30 Because our animals have incurred sleep loss, one might expect their sleep drive to be higher than control animals. However the normal number of sleep state entries indicates that the primary cause of sleep loss in this group is not lack of sleep drive, but rather that the VLPO apparatus for maintaining sleep had been damaged.
Effects of Chronic Partial Sleep Loss on Food Intake and Body Weight in Rats
Chronic partial sleep loss in VLPOx animals resulted in decelerated weight gain despite food intake equivalent to controls. Previous studies employing sleep deprivation in rodents have also reported slower weight gain (or even weight loss), but the reports of food intake were inconsistent. While most studies claimed an increase in food intake,31–34 others reported no changes in food intake after sleep loss.35–37 Martins and colleagues, in a well-controlled study,37 showed that the increase in food intake during sleep deprivation was in fact an artifact of food spillage rather than an actual increase. On the other hand, another study using liquid food where there was no spillage found an increase in food intake in animals sleep deprived for 10 days.38 However, these studies contained two confounds. First, the methods of sleep deprivation involved variable degrees of stress, and elevation of corticosteroids by itself can alter food intake.39,40 Second, most of these studies37,38 have been of relatively short duration. In our experiment, rats maintained chronic partial sleep loss throughout the experiment without any external intervention and they did not show a significant difference in food intake, despite exhibiting an increase in plasma ghrelin and a decrease in leptin levels similar to sleep-restricted humans.7 While these changes in ghrelin and leptin have been shown to be correlated with an increase in appetite and subsequent food intake in animals and humans that have not been sleep deprived,41–43 it appears that sleep loss in rats may blunt this effect. One possible interpretation of our data, which is easily testable, is that the responsiveness to ghrelin and leptin may represent a fundamental species difference between humans and rodents in response to sleep loss. An alternative interpretation is that our VLPO lesions may have damaged neurons necessary for the response to ghrelin. This is unlikely because the region of the VLPO contains very few neurons with ghrelin receptors44 and because failure to gain weight was seen in earlier studies that used other methods of sleep deprivation in rats.32–34 In addition, ghrelin-induced food intake has been shown to be primarily mediated by neuropeptide Y (NPY)/agouti-related peptide (AgRP) and proopiomelanocortin (POMC) neurons in the arcuate nucleus.45–47 Thus, it is unlikely that the lack of hyperphagia despite an increase in ghrelin levels in the VLPOx animals is due to loss of ghrelin-responsive neurons in the preoptic region.
On the other hand, neurons in and around the VLPO have been shown to project to the dorsomedial, paraventricular, and arcuate hypothalamic nuclei as well as the lateral hypothalamus,48 which play important roles in energy homeostasis. Although it is not clear which of these projections arise from the galaninergic VLPO neurons, as opposed to other cells in the region, it is reasonable to speculate that the lack of increased feeding in our study may have reflected the loss of neurons in the VLPO region with projections to the mediobasal hypothalamus. But, the lack of significant correlation between food intake and the percentage of cell loss in the entire VLPO region does not support this hypothesis. While this finding does not eliminate the possibility that VLPO neurons may contribute to some other aspect of metabolism (e.g., energy expenditure), it does suggest that VLPO neurons themselves have little impact on the amount of food consumed. In the future, genetically driven methods that would label projections only from the galaninergic neurons in the VLPO, or would kill only these sleep-active neurons, would be of great value in sorting out this issue.
On the other hand, our results are compatible with a range of other sleep deprivation paradigms in animals, which consistently show weight loss or slower weight gain during sleep deprivation.32,33,49,50 Recently, it has been shown that sleep deprivation for 40 hours in humans produced an increase in energy expenditure.51 This might also be true for chronic partial sleep loss in the animals with VLPO lesions. Although energy expenditure was not measured in the present study, we did find that the duration of active periods, as judged by EMG activity, were increased in our VLPOx animals. In a separate series of preliminary studies in which we recorded locomotor activity by telemetry, we found that locomotor activity in VLPOx rats was ∼40% higher than the control animals (Vetrivelan, Lu and Saper, unpublished observations), which could contribute to increased energy expenditure in these animals. But it is possible that they may have burned more calories in other ways as well, e.g., by brown adipose tissue thermogenesis. Consistent with this possibility, specific REM sleep deprivation has been shown to increase the gene expression of uncoupling protein-1 (UCP1; mediates the heat generation) in brown adipose tissue.49 Further studies directly measuring metabolic rate and brown adipose activation in VLPOx rats would be of great interest. Such studies could help to determine whether the decelerated weight gain in the VLPOx animals is due to increased energy expenditure, failure to increase food intake in concert with energy demands, or some combination of the two.
Sleep Loss and the Metabolic Syndrome
The metabolic syndrome, in general, is associated with an increase in adiposity, a decrease in insulin sensitivity, and an increase in proinflammatory factors. The VLPOx-10S animals demonstrated a decrease in glucose levels without a change in insulin levels, indicating that, although not measured directly, insulin sensitivity may be increased in these animals. In addition, higher adiponectin and lower leptin levels indicated a reduction in visceral adiposity in VLPOx animals. Finally, these animals also did not appear to be in a pro-inflammatory state, as evidenced from decreased plasma levels of CRP and increased levels of adiponectin. Collectively, VLPOx animals with chronic partial sleep loss demonstrated none of the plasma indicators of metabolic syndrome.
In contrast, chronic partial sleep loss in humans has been associated with an increased risk for obesity, T2D, and cardiovascular events.7,52–54 The apparent differential metabolic response(s) to chronic partial sleep loss between humans and rats may therefore simply reflect a species difference, although several additional and possibly contributing factors differ between our VLPOx rats and humans who voluntarily restrict their sleep. For example, whereas VLPOx rats did not consume more food than controls, sleep-restricted humans in short term experiments ate significantly more than control subjects and increased consumption of excess calories from snacks.55 Moreover, it has been suggested that humans may be less active during most of their extended waking time while the VLPOx rats with chronic sleep loss did not show a reduction in activity levels. In part this may be because the activities that humans choose in life (or are chosen for them in studies) during their extended wake time may be largely sedentary. As noted above, the difference in response of feeding and energy expenditure to elevated levels of ghrelin and low leptin, and possibly other factors, during sleep deprivation may represent a fundamental species difference between rats and humans.
On the other hand, in short duration studies in which both activity and food consumption have been controlled in healthy young humans subjected to partial sleep restriction, adverse metabolic consequences ranging from glucose intolerance to elevated levels of CRP were still observed. One explanation for this difference may be that the methods used for sleep deprivation may be inherently stressful. For example, experimental sleep restriction (generally involving social interaction) in humans produced an increase in cortisol levels.56 Alternatively, all of these studies involve circadian shifts of one type or another in wake-sleep behavior (i.e., subjects either go to sleep late, wake up early, or both). Although the subjects are held in low light conditions to avoid light-mediated shifts in circadian rhythm, the possibility exists that the alterations of circadian rhythms of activity or feeding may contribute to the elevated appetite and metabolic dysregulation seen in humans. In animals and humans, circadian dysregulation has been shown to produce alterations in energy metabolism and may lead to obesity and diabetes.13,14,57
By contrast, the animals with VLPO lesions have no detectable change in circadian phase or period in sleep-wake (Figure 3) or body temperature,17 and the proportion of waking time spent in the light vs. the dark period did not differ significantly from the control animals. However, it could be argued that the lack of the metabolic syndrome following chronic sleep loss in the VLPOx animals was due to the smaller amount of sleep loss observed during their rest period (∼13%) when compared to that during their active period (∼44%). Although we are not aware of any studies investigating differential physiological/behavioral consequences of sleep deprivation during the day vs. night in rodents, sleep deprivation during either period has produced similar responses in terms of sleep homeostasis—slow wave energy attained during the sleep rebound was similar after sleep deprivation either during the day or night.30,58 In fact, the magnitude of sleep increase in rats was greater after sleep deprivation at night compared to sleep deprivation during the day,58 indicating that sleep at night in nocturnal animals may be as important as sleep during the daytime. Thus, differences in metabolic consequences following chronic sleep loss in humans and VLPOx rats are unlikely to be due to lesser sleep loss during their rest phase than during their active phase.
Collectively, our data suggest that VLPOx animals may be a useful model to study the consequences of chronic partial sleep loss, but without stressful interventions or circadian rhythms disruption. Our findings from this model strongly suggest that chronic partial sleep loss per se does not lead to obesity or metabolic syndrome, at least in rats. This finding raises the question whether chronic partial sleep loss in humans may cause adverse metabolic consequences due to other factors such as circadian disruption and the types of activities that are maintained during the extra waking hours, particularly increased feeding and decreased activity levels.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants AG09975, NS072337, and HL095491. We thank Quan Ha for excellent technical assistance and Drs. Dong Kong and Andrew Lim for helpful discussions. We are also thankful to the Specialized Assay Core at Joslin Diabetes Center (5P30-DK-36836) for the biochemical assays. Work for this study was performed at Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, MA.
Footnotes
A commentary on this article appears in this issue on page 1447.
REFERENCES
- 1.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 4.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000;24:1683–8. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31:645–52. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 10.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr. 2007;27:219–40. doi: 10.1146/annurev.nutr.27.061406.093546. [DOI] [PubMed] [Google Scholar]
- 14.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 16.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 19.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 ed. Elsevier; 2004. [DOI] [PubMed] [Google Scholar]
- 22.Elmquist JK, Breder CD, Sherin JE, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–29. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol. 1998;274:R783–9. doi: 10.1152/ajpregu.1998.274.3.R783. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–76. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- 26.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.2009 Sleep in America Poll summary of findings. cited; Available from: http://www.sleepfoundation.org/sites/default/files/2009%20Sleep%20in%20America%20SOF%20EMBARGOED.pdf.
- 29.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–64. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–33. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:E1060–70. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. 1989. Sleep. 2002;25:68–87. [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–4. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 35.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–6. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 36.Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav. 1999;67:643–9. doi: 10.1016/s0031-9384(99)00120-1. [DOI] [PubMed] [Google Scholar]
- 37.Martins PJ, D'Almeida V, Nobrega JN, Tufik S. A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep. 2006;29:1233–8. doi: 10.1093/sleep/29.9.1233. [DOI] [PubMed] [Google Scholar]
- 38.Koban M, Sita LV, Le WW, Hoffman GE. Sleep deprivation of rats: the hyperphagic response is real. Sleep. 2008;31:927–33. [PMC free article] [PubMed] [Google Scholar]
- 39.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–65. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–8. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 41.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 42.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 43.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology. 2003;144:3749–56. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 44.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 47.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;289:E68–74. doi: 10.1152/ajpendo.00543.2004. [DOI] [PubMed] [Google Scholar]
- 50.Caron AM, Stephenson R. Energy expenditure is affected by rate of accumulation of sleep deficit in rats. Sleep. 2010;33:1226–35. doi: 10.1093/sleep/33.9.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 53.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull. 2007;74:37–44. doi: 10.1016/j.brainresbull.2007.05.001. [DOI] [PubMed] [Google Scholar]