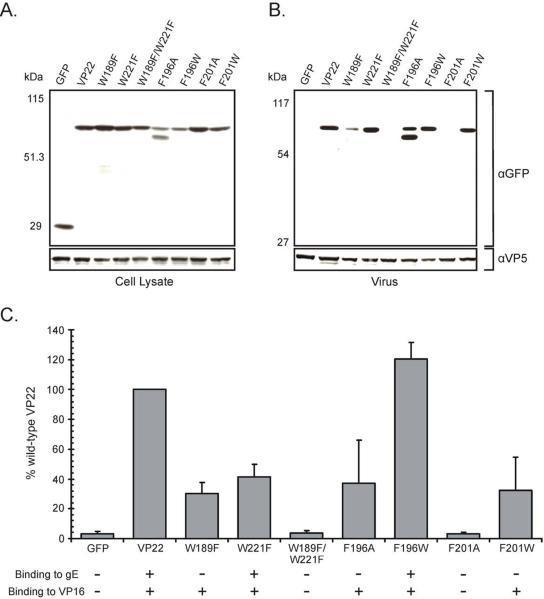
Figure 4. Virion incorporation of VP22 W→F and F→A/W point mutants in the absence of virally encoded VP22.
Vero cells were transfected with the indicated VP22-GFP constructs, and 20 h later, they were infected with a VP22-null virus [UL49−]. After an additional 18-h incubation, cell lysates were prepared (A) and virions were collected from the media by centrifugation through a 30% sucrose cushion (B). Cell lysates and extracellular virus were separated by SDS-PAGE and transferred to nitrocellulose. Western blot analysis was performed using a rabbit polyclonal antibody specific for GFP. As a loading control, the blot was stripped and reprobed with a rabbit monospecific polyclonal antibody raised against the HSV-1 major capsid protein VP5. The positions of molecular mass markers (in kilodaltons) are indicated on the left. (C) Packaging efficiency. Using densitometry, packaging efficiency was quantitated by dividing the amount of VP22-GFP protein detected in extracellular virus particles (normalized for VP5) by the amount in the cell lysate (normalized for VP5). In each experiment, the wild-type VP22-GFP construct was set at 100% packaging efficiency. Error bars represent standard deviations for four replicate experiments. The ability of each VP22 mutant to bind to either gE (as determined in Fig. 1) or VP16 (as determined in Fig. 2 and Fig. 3) is also represented. A plus indicates that the mutant construct retains binding activity, whereas a minus denotes abrogation of binding.