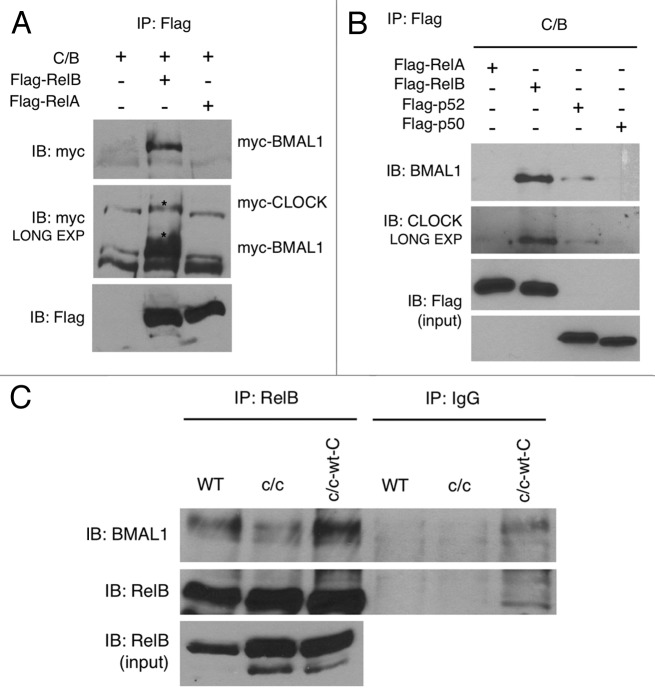
Figure 3. RelB interaction with BMAL1 and CLOCK. (A) HEK-293 cells were cotransfected with Myc-CLOCK and Myc-BMAL1 (C/B), without or with Flag-RelB or Flag-RelA. Flag-tagged proteins were immunoprecipitated by FLAG-Agar, and abundance of coimmunoprecipitated proteins was determined by western blotting with anti-Myc antibody and anti-Flag antibody. Asterisks indicate specific signals for BMAL1 and CLOCK proteins. (B) HEK-293 cells were cotransfected with Myc-CLOCK and Myc-BMAL1 (C/B) and series of expression vectors as described. Total lysates were prepared and subjected to immunoprecipitation using FLAG-Agar and coimmunoprecipitated proteins were detected by western blotting with anti-BMAL1 and anti-CLOCK antibodies. Lower panels show the expression of Flag-tagged proteins in total cell lysates as an input. (C) Cell extracts prepared from wild-type (WT), Clock mutant (c/c) and Clock mutant stably transfected with wild-type Myc-CLOCK (c/c-wt-C) were immunoprecipitated with RelB antibody or normal IgG, and immunoprecipitated BMAL1 and RelB were detected by probing with the BMAL1 and RelB antibody, respectively. Lower panel shows RelB expression in total cell lysates as an input.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.