Abstract
A tsunami affected area in Phang Nga province, Thailand was explored randomly as some freshwater sites had changed into brackish-water sites. A survey of four areas found Culex sitiens to be the most dominant mosquito species.This mosquito prefers to breed in putrefied water with garbage and it was found in almost every stagnant, brackish-water site in full sunlight. The larval density was more than 300 larvae/dip/250 ml water. Its biting cycle, determined by human landing catch, was nocturnal, with a single peak at 19.00–20.00 hr. The maximum rate was 108 mosquitoes per person/hour. The biology of the mosquito was studied by colonization in natural water under laboratory conditions. The mean number of eggs per raft was 158.1 ± 31.7, hatchability 96.6 ± 4.1%, development from 1st instar larvae to adult was 8.8–11.7 days, and longevity of adult males was 7.3–41.3 days and females 11.0–52.7 days. The ratio of adult males to adult females was 1:1.1 ± 0.2.
Keywords : Culex sitiens; filariasis vector; biting cycle; breeding habitats; colonization; longevity
Introduction
In 2009 a tsunami hit the Andaman Coast, which is 954 kilometers in length in the southern part. Areas almost two or three kilometers from the coastline were devastated by the power of the waves, resulting in several brackish-water sites (Wattanawaitunechai et al. 2005). These new ponds containing salt marshes are potential breeding sites for mosquito vectors. Some of the freshwater sites have changed into brackish-water sites due to being filled with seawater. Larvae of Culex sitiens Weidemann (Diptera: Culicidae) and Anopheles sundaicus Rodenwaldt have been observed a year after the disaster in every water site at moderate to high densities of 40–50 larvae/dip to >100 larvae/dip (250–300ml container). A nuisance biting mosquito species was observed as 42 mosquitoes per man per 10 minutes at 19.00 hr (Apiwatthnasorn et al 2005). This result agreed with the reports of the local people in the affected areas. Apiwatthnasorn et al. (2005) also discussed the irregular increase in the mosquito population compared with the pre-tsunami period.
Even though there has been no report of an epidemic of mosquito vector-borne diseases after the tsunami, a concern was that local Myanmar laborers, who are considered as carriers of Japanese encephalitis and filariasis, might initiate a local resurgence of disease. Human filariasis is caused by the nematodes, Brugia malayi Brug (Spirurida: Onchocercidae) and Wuchereria bancrofti and it is a public health problem in the southern part of Thailand. Filariasis is a zoonotic infection that is vectored by mosquitoes. It is endemic in Narathiwat, Nakhon Si Thammarat, Surat Thani and Krabi provinces (Phantana et al. 1978). Japanese encephalitis is caused by a virus that infects the nervous system. It is common in Thailand (Umenai et al. 1985; Solomon et al. 2000) and is transmitted from birds and pigs to humans by Culex mosquitoes. Cx .siitens was found to be completely refractory to experimental infection with B. malayi. Despite the presence of high larval and biting densities, it appears to play no role in B. malayi transmission in the southern part of Thailand (Prummongkol et al. 2009). Iyengar (1953) reported that Cx. sitiens showed a few filaria larvae in a very early stage of development, but the larvae showed signs of degeneration, such as distortion, swelling, and vacuolation, indicating that the infection was not likely to develop any further. Cx. sitiens is a potential vector for Japanese encephalitis (Vythilingam et al. 2002). An. sundaicus is a malaria vector in coastal areas of Thailand and other countries (Chowanadisai et al. 1989).
The biology of Cx. sitiens, a possible vector in tsunami affected areas, including their breeding habitat, biting activity was determined so that it could be investigated and monitored a new prevention and control measure strategy in the Phang Nga area.
Materials and Methods
A survey in the post-tsunami affected areas was explored in a random fashion during February to April 2005. Some freshwater sites had changed into brackish-water sites. The denatured and polluted water sites that formed had become breeding areas for Cx. sitiens. The collection stations were set at Phang Nga Naval Base, Ban Keuk Kuk, Ban Bang Ka Ya and Ban Nam Khem, Phang Nga province.
Natural breeding habitats
Surveys of breeding places of Cx. sitiens were carried out and mosquito species and aquatic predators were determined as well as some quality parameters of the breeding water, such as pH, conductivity, total dissolved solids, dissolved oxygen, salinity and temperature. The water quality was recorded using a Sension 156 Portable Multiparameter Meter (HACH, www.hach.com). Identification of the collected mosquitoes was based on adults emerging from the pupae.
Biting cycle
Collection stations were established at all study sites to determine the biting cycle of the mosquito studies. The biting cycles were conducted outdoors between 1800–2400 hours, by human landing catch. Mosquito counts were made hourly for 45 minutes by two groups, each with two persons, for three periods of time during 3 consecutive nights from Nov. to Dec. 2005. All mosquitoes were identified as to species by using a standard dissecting microscope and taxonomic keys (Bram 1967; Ratanarithikul et al. 2005). Female Cx. sitiens mosquitoes were transferred into paper cups with screen tops (approximately 50 mosquitoes per cup) and cotton wool soaked with 10% sugar solution was offered as a food. These cups were placed in a cooler covered with a wet towel. Ice packing was used to keep them cool and provide humidity during transportation.
Biology Laboratory colonization of Culex sitiens
The mosquitoes were reared individually to get single colonies using a modified procedure (Panicker et al. 1981; Limsuwan et al. 1987). After returning to the laboratory, the female mosquitoes were released into a 30×30×30 cm cage as starting colonies and the females were given a blood meal from a golden hamster by placing it inside a mosquito cage overnight. The engorged females were transferred into paper cups (approximately 15 individuals per cup) and cotton wool soaked with 10% sugar solution was offered as food. About three or four days after feeding, each mosquito was transferred to a plastic cup containing 15 ml natural water which was carried from the field at the collecting areas for oviposition. Egg rafts were separated individually in plastic cups with the same natural water to observe hatchability. On the following day, the number of eggs that hatched were scored from 20 egg rafts. The number of eggs in each raft, the duration of different larvae instars, pupae and adults recorded every day. The larvae were reared in a plastic trays with about 1000 ml of natural water (from field study area) per tray. A mixture of Powered Fish 2000 (White Crane Aquarium Co. Ltd. (www.whitecranev88.com) and water was provided as larval food. Food was added to each plastic rearing tray at each of the 4 instars, 0.5, 1.0, 1.5 and 2.0 ml respectively. Numbers of male and female mosquitoes that emerged were counted and recorded, cotton wool soaked with 10% sugar solution was provided as food for the adults. The insectarium used was not air-conditioned, the temperature and relative humidity were recorded and measured. The rearing temperature was 30.8 ± 0.5°C (range 29–31°C) with approximately 68.2 ± 3.1% (64–70%) relative humidity.
Observations on longevity
The longevity of eggs, larvae, pupae and adults was assessed. Thirty of each of the newly emerged males and females were kept separate in paper cups covered with screen tops, with cotton wool soaked with 10% sugar solution provided as food. These sugar pads were provided regularly and changed every day until the adults died. Paper cups were checked daily and dead mosquitoes were counted and removed until all of mosquitoes died. The sex ratio of the adult male and female was also determined.
Results and Discussion
Nature characteristics of breeding habitats
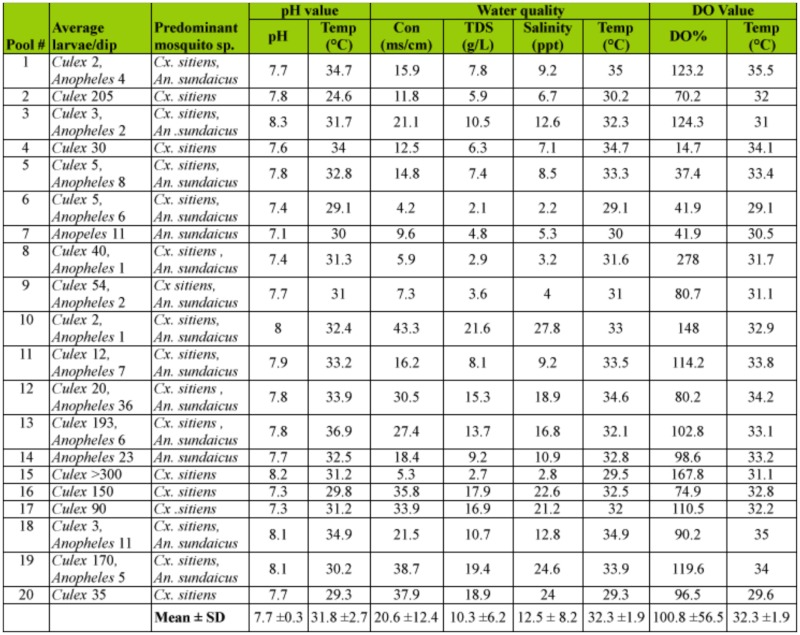
Mosquito larvae were collected from breeding habitats in the tsunami-affected areas of Phang Nga province, from Nov. to Dec. 2005 and the results are shown in Table 1. The immature stages of Culex sitiens inhabit sunlit stagnant water, including small pits, pit lakes, wells, swamps, ponds, canals, particular shrimp ponds and water that collects in abandoned tin mines, as in previous studies (Apiwathnasorn et al. 2005). Their breeding habitats varied in size from small pits (1×2 m2) to large water reservoirs such as pit lakes or water collections in abandoned tin mines (220×730 m2). The water may be contaminated with garbage, floating pieces of dried leaves, filamentous green algae (Enteromorpha sp.) and grasses. In some breeding places fishes, naiads and shrimp were found. It was found that almost all sites of breeding places were positive for Cx. sitiens larvae with a maximum larval density of more than 300 larvae per dip (water volume 250 ml). The water quality of breeding habitats is shown in Table 1. The mean salinity was 12.5 ± 8.2 ppt.
Table 1.
Larval survey and water quality in the observation breeding places in the tsunami affected area, Phang Nga Province, Nov–Dec 2005.
Three larvae mosquito species were found with breeding habitats in tsunami affected areas, Phang Nga province. Cx. sitiens have been described as a brackish-water breeding mosquito and are generally regarded as being restricted to costal regions (Bram 1967; Bick 1951; Colless 1957c). In our study we found that Cx. sitiens preferred to breed in more putrefied water with garbage as previously reported (Apiwathnasorn et al. 2005). Cx. sitiens was the most dominant species, but An. sundaicus and Aedeomyia catasticta Knab were also recorded.
Biting activity of mosquito
The biting cycle of Cx. sitiens was determined by human landing catch. There were 10 mosquito species collected from 4 stations mentioned above during the biting cycle study. These were Cx. sitiens, Cx. gelidus, Cx. quinquefasciatus, Mansonia uniformis, An. sundaicus, An. vagus, Aedes albopictus, Ae. aegypti, Ochlerotatus vigilax, and Armageres subalbatus. Cx. sitiens was the most abundant species collected from 4 stations. A total of 5,095 mosquitoes were collected by human landing catches. The most abundant species was Cx. sitiens 1,434 (81.1%), An. sundaicus 451 (8.9%), Cx. quinquefasciatus 367 (7.2%), Cx. gelidus 78 (1.5%), Ar. subalbatus 27 (0.5%), Oc. vigilax 11 (0.2%), An. vagus and Ma. uniformis 9 (0.2%), Ae. albopictus 8 (0.2%) and Ae. aegypti 1 (0%) (Figure 1).
Figure 1.
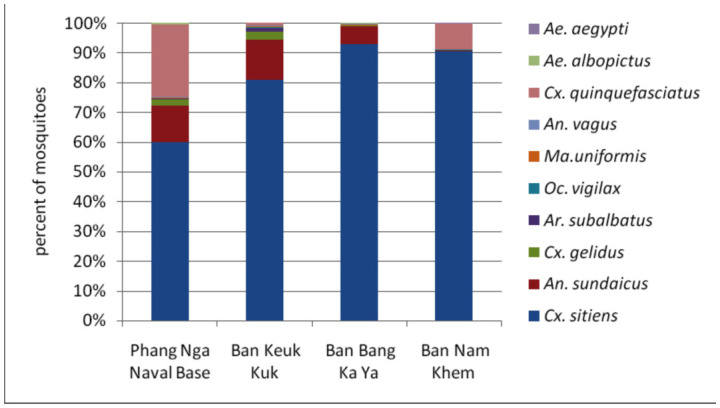
Percentage of mosquito species by human landing catch Nov–Dec 2005. High quality figures are available online.
Cx. sitiens has been reported in Thailand and in neighboring countries including India, Indonesia, Malaysia, Vietnam, Myanmar and Cambodia (Chowanadisai et al. 1989; Harinasuta 1974; Chow 1970). It was previously regarded as a secondary vector of malaria in Thailand (Chowanadisai et al. 1989; Chow 1970; Rattanarithikul and Panthusiri 1994). Cx. quinquefasciatus is the vector of W. bancrofti urban type (Burke and Leake 1988). Cx. gelidus is the vector of Japanese encephalitis (Burke and Leake 1988). Ar. subalbatus is a potential vector of B. pahangi in experimental studies in Thailand and Malaysia (Sucharit and Sucharit 1988). Oc. vigilax appears to be the principal vector of non-periodic filariasis in New Caledonia (Iyengar 1954) and has been found naturally infected with Murray Valley encephalitis virus in Australia (Doherty et al. 1963). Ma. uniformis was incriminated as a vector of brugian filariasis (Harinasuta et al. 1970). The susceptibility of An. vagus to nocturnally subperiodic B. malayi was studied in the laboratory and the results showed that the infective rate was only 0.74% because they have well developed cibarial armatures that damage 94.57% of ingested microfilaria (Choochote et al. 1987).
The biting cycle of Cx. sitiens mosquito which was determined by human landing catch was nocturnal, with a single peak at 19.00–20.00 hr (Figure 2). The maximum biting rate of Cx. sitiens was 108 mosquitoes per person-hour, similar to the result from Apiwathnasorn et al. (2005), when 135 mosquitoes per person-hour were reported. Their biting densities were very high at 108, 64, 46, and 33 bites per person-hour at peak periods in Ban Keuk Kuk, Ban Nam Khem, Ban Bang Ka Ya, and Phang Nga Naval Base, respectively.
Figure 2.
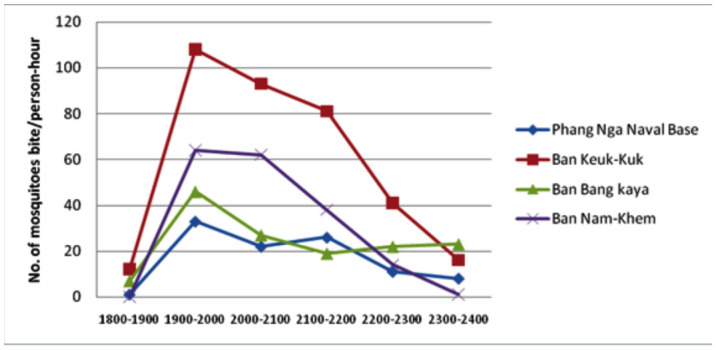
Overall biting activity of Culex sitiens mosquitoes at 1800–2400 hr, combining biting cycles from 4 collecting stations Nov–Dec 2005. High quality figures are available online.
Mosquito colonization
Each raft of eggs was hatched in natural water from the breeding habitat, which had a pH 7.4 (temperature 32.1°C), conductivity 5.7ms/cm, total dissolved solid 2.9g/l, salinity 3.1ppt (32.3°C) and dissolved oxygen 60.4% (32.6°C). Based on the ecology and biology of Cx .sitiens obtained from this study, it was successfully colonized and could be consecutively colonized in insectariums at a temperature of 30.8 ± 0.5°C (range 29–31°C) and 68.2 ± 3.1% (range 64–70%) relative humidity.
Observations of the number of egg per raft, hatchability, immature survival and sex ratio were made. The mean number of eggs in each raft was 158.1 ± 31.7 (range 104–223). The egg stage lasted for 2–3 days and the larvae emerged through a transverse slit at the anterior end of the egg. Among deposited egg rafts, all of them were viable with average hatchability 96.6 ± 4.1% (range 82.3–100%). The life duration of different larval instars and pupa was studied by measuring immature survival from 1st instar to pupa stage and was 92.2 ± 12.1% (range 55.3–100%). The ratio of adult males to adult females was 1:1.1 ± 0.2.
The duration of different larval instars, pupa and adult were studied and the results showed that the mean range of duration of the first, second, third and fourth larval instars were 3.2 ± 1.6, 5.2 ± 2.2, 6.6 ± 1.4 and 7.6 ± 1.4 days, respectively. Then at 8.9 ± 1.6 days they became pupae and emerged as adults at 10.3 ± 1.4 days.
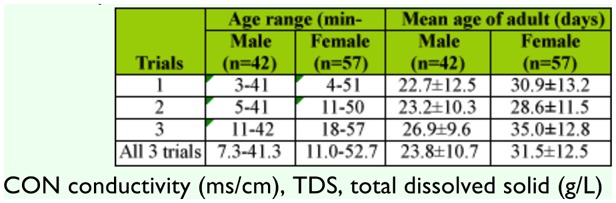
Observations on the duration of thirty adult males and females determined in laboratory rearing are shown in Table 2. Three trials of longevity were performed. Male Cx. sitiens fed 10% sugar solution, survived for 7.3–41.3 days, while females survived for 11.0–52.7 days.
Table 2.
Longevity of adult males and females Culex sitiens under laboratory conditions.
The ratio of adult males to adult females was 1:1.1 ± 0.2. The duration of different larval instars, pupa and adult were studied and the results showed that the mean range of duration of the first, second, third and fourth larval instars are 3.2 ± 1.6, 5.2 ± 2.2, 6.6 ± 1.4 and 7.6 ± 1.4 days, respectively. Then at 8.9 ± 1.6 days they became pupae and emerged as adults at 10.3 ± 1.4 days.
Conclusion
A large-scale environmental change has been observed post-tsunami period in tsunami affected areas of Phang Nga province. The ecology and biology of Cx. sitiens; such as their breeding habitat and biting activity were studied, and there were increasing numbers of Cx. sitiens. Cx. sitiens was the dominant mosquito species. The maximum biting rate was 108 mosquitoes per person-hour. It has been described as a brackish-water breeding mosquito but it was found that they preferred to breed in more putrefied water with garbage. The duration of different larval instars, pupa and adult were studied and the results showed that the mean of duration from larvae to adult was about 11 days.
Acknowledgements
We gratefully acknowledge the help and support provided by staffs of the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. We thank Prof. Dr. Alister Craig, the Liverpool School of Tropical Medicine, Liverpool, UK, for his kind reading and gave very helpful comments to this MS. We would also like to thank Eric Curkendall of the Office of International Relations, Faculty of Public Health, Mahidol University for his help editing this article. This work was also partially supported by the China Medical Board, Faculty of Public Health, Mahidol University, Bangkok, Thailand (UL).
References
- Annual Report of the Bureau of Vector-Borne Disease. Department of Disease control, Ministry of Public Health. 2004:57–70. [Google Scholar]
- Apiwathnasorn C, Samung Y, Prummongkol S. Impact of tsunami on occurrence of mosquito vectors in the affected areas of Phang Nga province. Proceeding: Joint International Tropical Medicine Meeting 2005.
- Bick GH. The ecology of the mosquito larvae of New Guinea. Pacific Science. 1951;4:392–431. [Google Scholar]
- Bram RA. Contributions to the mosquito fauna of Southeast Asia. II. The genus Culex in Thailand (Diptera: Culicidae). Contributions of the American Entomological Institute. 1967;2:239–247. [Google Scholar]
- Burke DS, Leake CJ. Japanese encephalitis. In: Monath TP, editor. The arboviruss: epidemiology and ecology. III. CRC Press; 1988. pp. 63–92. [Google Scholar]
- Choochote W, Sucharit S, Khamboonruang C, Vuthikes S, Surathin K, Deesin T, Keha P. Determination of the susceptibility of Anopheles mosquitoes to nocturnally subperiodic Brugia malayi (Narathiwat, southern Thailand). Journal of Parasitology and Tropical Medicine Association of Thailand. 1987;10:1–8. [Google Scholar]
- Chow CY. Bionomic of malaria vectors in the western Pacific region. Southeast Asian Journal of Tropical Medicine and Public Health. 1970;1:40–57. [Google Scholar]
- Chowanadisai L, Krairiksh S, Thanasripukdikul S. Microbial control of Anopheles sundaicus, a malaria vector in Thailand, by Bacillus sphaericus 2360. Mosquito Borne Diseases Bulletin. 1989;6:39–44. [Google Scholar]
- Colless DH. Notes on the culicine mosquitoes of Singapore. Larval breeding places. Annals of Tropical Medicine and Parasitology. 1957c;51:102–106. doi: 10.1080/00034983.1957.11685799. [DOI] [PubMed] [Google Scholar]
- Doherty R, Carly G, Mackerras MJ, Marks EN. Studies of arthropod-borne virus infections in Queensland. Isolation and characterization of virus strains from wildcaught mosquitoes in north Queensland. Australian Journal of Experimental Biological and Medical Sciences. 1963;41:17–40. doi: 10.1038/icb.1963.2. [DOI] [PubMed] [Google Scholar]
- Harinasuta C, Charoenlarp P, Sucharit S, Deesin T, Surathin K, Vuthikes S. Studies of Malayan filariasis in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1970;1:29–39. [Google Scholar]
- Harinasuta C, Guptavanij P, Vasuvat C. Studies on the medical ecological epidemiology in mangrove areas in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1974;5:105–27. [PubMed] [Google Scholar]
- Iyengar MOT. Filariasis in Thailand. Bulletin of World Health Organization. 1953;9:731–766. [PMC free article] [PubMed] [Google Scholar]
- Iyengar MOT. Distribution of filariasis in the South Pacific region. South Pacific Commisson Technical Paper. 1954;66:1–52. [Google Scholar]
- Limsuwan S, Rongsriyam Y, Kerdpibule V, Apiwathanasorn C, Chang GL, Cheong WH. Practical entomology malaria and filariasis. Siriyod Co., Ltd; 1987. Rearing techniques for mosquitoes. ; pp. 47–54. [Google Scholar]
- Mosha FW, Magayuka SA. Potential vectors and non-vectors of bancroftian filariasis in East Africa. East African Medical Journal. 1979;56:197. [PubMed] [Google Scholar]
- Panicker KN, Bai Geetha M, Viswam K. Laboratory studies on the biology of Culex sitiens Wiedemann, 1828. Indian Journal of Medical Research. 1981;73:147–150. [PubMed] [Google Scholar]
- Phantana S, Shutidamrong C, Chusattayanond W. Brugia malayi in a cat from southern Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978;81:173–174. doi: 10.1016/0035-9203(87)90330-0. [DOI] [PubMed] [Google Scholar]
- Prummongkol S, Panasoponkul C, Apiwathnasorn C, Lek-Uthai U. Refractoriness of Cx. sitiens to experimental infection with nocturnal subperiodic Brugia malayi. Journal of Tropical Medicine and Parasitology. 2009;32:82–86. [Google Scholar]
- Ratanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. Illustrated keys to the mosquitoes of Thailand. Genera Culex and Lutzia. Southeast Asian Journal of Tropical Medicine and Public Health. 2005;36:1–47. [PubMed] [Google Scholar]
- Rattanarithikul R, Panthusiri P. Illustrated keys to the medical important mosquitoes of Thailand. Southeast Asian Journal of Tropical Medicine and Public Health Supplement 1. 1994:1–66. [PubMed] [Google Scholar]
- Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. Journal of Neurological and Neurosurgical Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharit S, Sucharit P. Medical Entomology. 1st ed. Bangkok: Krungthepkarnpim; 1988. pp. 260–262. 640. [Google Scholar]
- Umenai T, Krzysko R, Bektimirov TA, Assaad FA. Japanese encephalitis: current worldwide status. Bulletin of the World Health Organization. 1985;63(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- Vythilingam I, Tan SB, Krishnasamy M. Short Communication: Susceptibility of Culex sitiens to Japanese encephalitis virus in peninsular Malaysia. Tropical Medicine and International Health. 2002;7:539–540. doi: 10.1046/j.1365-3156.2002.00896.x. [DOI] [PubMed] [Google Scholar]
- Wattanawaitunechai C, Peacock SJ, Jitpratoom P. Tsunami in Thailand-Disaster Management in a District Hospital. New England Journal of Medicine. 2005;325:962–964. doi: 10.1056/NEJMp058040. [DOI] [PubMed] [Google Scholar]