Abstract
Vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) remains the only prophylactic vaccine against tuberculosis, caused by Mycobacterium tuberculosis (Mtb), but gives variable protection against pulmonary disease. The generation of host Th1 responses following BCG vaccination is accepted as the major mechanism of protection against Mtb infection. Early production of IL-17 in the lungs following Mtb challenge of mice previously vaccinated with Mtb peptides in adjuvant has been shown to be required for efficient Th1 cell recruitment. IL-10 regulates various processes involved in generation of Th1 and Th17 responses. Previous studies have shown IL-10 as a negative regulator of the immune response to primary Mtb infection, with Il10−/− mice having reduced lung bacterial loads. In this study we show that inhibition of IL-10 signaling during BCG vaccination enhances host-generated antigen-specific IFN-γ and IL-17A responses, and that this regime gives significantly greater protection against aerogenic Mtb challenge in both susceptible and relatively resistant strains of mice. In Mtb-susceptible CBA/J mice, antibody blockade of IL-10R specifically during BCG vaccination resulted in additional protection against Mtb challenge of greater than 1-Log10 compared to equivalent isotype-treated controls. Protection observed following BCG vaccination concurrent with anti-IL-10R mAb treatment was sustained through chronic Mtb infection, and correlated with enhanced lung Th1 and Th17 responses, and enhanced IFN-γ and IL-17A production by γδ T cells and an innate-like Thy1.2+CD3— lymphoid population. We show that IL-10 inhibits optimal BCG-elicited protection, therefore suggesting antagonists of IL-10 may be of great benefit as adjuvants in preventive vaccination against tuberculosis.
Introduction
Tuberculosis (TB), caused by the intracellular pathogen Mycobacterium tuberculosis (Mtb), remains a major global threat to humanity accounting for 8.8 million new cases per year and over 1.4 million deaths worldwide (1). The global burden of TB is fuelled by emergence of multi-drug-resistant Mtb strains (2) and the variable protection given by the only current vaccine against pulmonary TB, M. bovis bacillus Calmette-Guérin (BCG) (3-5). In light of this, substantial efforts have been made to develop better TB vaccines, with several new vaccination strategies in development (3). However, the design of new vaccines against TB is hampered by the lack of correlates of protective immunity, and the need for a better understanding of the immune response to Mtb infection (3, 5, 6). An additional complexity is the estimation that one third of the world’s population may have latent infection, with an associated 10-20% lifetime risk of progression to active disease (7); how this may impact vaccination is as yet unclear (3, 8).
Immune control of Mtb infection is known to require TNF-α (9, 10) and IFN-γ (11-13); the latter cytokine produced by a robust Th1 cell-mediated response that in turn requires IL-12 for its generation in mouse and human (6, 13-15). IL-1α/β has also been shown to be a critical protective factor for the host during experimental Mtb infection of mice (16-18). Current vaccination strategies aim to create enhanced Th1 memory responses that direct macrophage killing of Mtb. Effective control of pulmonary Mtb infection is also likely to require efficient localization of Th1 responses to the lung, and in a timely enough manner to control the pathogen (6, 19-22). Vaccination with Mtb peptide in adjuvant in relatively Mtb-resistant C57BL/6 mice results in early recruitment of Th1 memory cells in response to aerosol Mtb challenge, and has been shown to be dependent on production of IL-17 in the lung which induces T cell chemokines (23). More recent studies have proposed that IL-17 responses following BCG vaccination also contribute to vaccine-elicited Th1 immunity and protection to Mtb challenge (24). In contrast, repeated BCG vaccination of previously Mtb-infected mice results in IL-17-driven lung immunopathology associated with large-scale neutrophilia (25), raising the need for caution and a further understanding of the role that IL-17 plays in vaccination against Mtb infection (26, 27).
IL-10 regulates the immune response induced by various pathogens and their products, thereby preventing damage to host tissues (28). However, with some infections IL-10 impedes the ability of the host immune response to eliminate the pathogen, contributing to chronic infection (29-32). We and others have shown IL-10 to be a negative regulator of the immune response to primary Mtb infection in vivo without overt evidence of immunopathology in relatively Mtb-resistant and Mtb-susceptible mice (33-37). In our previously published study, in the absence of IL-10 signaling, mice showed greater control of bacterial load in lungs and spleens as compared to wild type (WT) control animals, corresponding with earlier and enhanced cytokine levels in these organs and in the serum (36).
The potential for IL-10 to similarly regulate the response to immunization has been demonstrated by the observation that neutralization of IL-10 during priming with soluble OVA protein in the presence of LPS leads to an enhanced Th1 response upon re-challenge (38). In models of infectious disease, inhibition of IL-10 signaling during vaccination against Leishmania major significantly decreases parasite burden and inflammation over vaccination alone (39-42). In established lymphocytic choriomeningitis virus infection, blockade of IL-10 receptor (IL-10R) signaling during an otherwise ineffective therapeutic DNA vaccination resulted in enhanced clearance of infection by increasing numbers of multifunctional virus-specific T cells (43). In mycobacterial infection, anti-IL-10R mAb administered before vaccination with Mtb culture filtrate protein (CFP) enhanced the immunogenicity of CFP, without requirement for additional adjuvant, and gave the vaccine the ability to protect against intravenous challenge with Mycobacterium avium (44). Another study has shown that systemic BCG infection of C57BL/6 Il10−/− mice resulted in lower bacterial burdens than WT controls (45). It has been suggested that blockade of IL-10 signaling might enhance BCG-elicited protection; since, at 30-days post Mtb challenge, BCG-vaccinated C57BL/6 Il10−/− mice have decreased bacterial burdens compared to WT BCG-vaccinated animals (24). However, given that C57BL/6 Il10−/− mice display decreased lung and spleen bacterial burdens beyond 28-days post aerogenic Mtb infection in the absence of vaccination (36), it is unclear using C57BL/6 Il10−/− mice whether IL-10 has regulatory effects both during Mtb challenge and vaccination, or whether IL-10 has a regulatory role specifically at the level of initial vaccination as has been shown in other models of infectious disease (38-42).
In the present study we have found that inhibition of IL-10 signaling during BCG vaccination enhances Th1 and Th17 responses, and IFN-γ and IL-17A production by CD8+ T cells, γδ T cells, and an innate-like Thy1.2+CD3— population in vivo. This resulted in significantly increased protection against aerogenic challenge with Mtb, compared to BCG vaccination alone. Furthermore, this protective response is sustained during the course of chronic Mtb infection, in both Mtb-susceptible CBA/J mice as well as in the relatively resistant C57BL/6 mouse strain, as compared to BCG vaccination alone. We also highlight the key lymphoid sources of the cytokines IFN-γ and IL-17A that correlate with the strongest level of protection against Mtb challenge in BCG-vaccinated/anti-IL-10R-treated mice.
Materials and Methods
Animals
Female C57BL/6 and C57BL/6 Il10−/− mice (8-12 wk) were bred and housed in the specific pathogen-free facilities at the Medical Research Council National Institute for Medical Research (NIMR, London, UK). Female CBA/J mice (8-10 wk) were obtained from The Jackson Laboratories (Bar Harbor, USA). Experiments were reviewed by and in accordance with the Home Office regulations (UK).
BCG vaccination and Mtb infection
Experiments were performed under Containment level-3 conditions. BCG Vaccine Danish 1331 (SSI, Denmark) and Mtb H37Rv were grown in Middlebrook 7H9 broth supplemented with 10% oleic acid albumin dextrose complex (OADC) (Difco), 0.05% Tween-80, and 0.5% glycerol to mid-log phase before freezing at −80°C. For vaccination, mice received 5 × 105 colony-forming units (CFU) BCG intradermally (i.d.) in Dulbecco’s PBS (Gibco), or PBS alone. For Mtb infections, 2 × 107 CFU Mtb H37Rv in PBS were aerosolized using a three-jet Collision nebulizer unit (BGI, USA) over a period of 15 min with approximately 30 CFU delivered to the lungs as confirmed by enumeration of bacteria on day 1 post-infection.
Anti-IL-10R mAb treatment
One day prior to BCG vaccination, mice were injected intra-peritoneally (i.p) with 1 mg of either anti-IL-10 receptor (IL-10R) mAb (a kind gift from DNAX, now Merck, Palo Alto, USA; 1B1.3A) that specifically binds the ligand-binding domain of IL-10R (46), or with IgG1 isotype control mAb (Merck; GL113) (46). Following vaccination mice received 0.35 mg of the respective mAb i.p., weekly for 6 weeks.
Processing of organs for cell culture and determination of bacterial burden
Lungs and spleens were aseptically removed from mice after sacrifice, placed into RPMI 1640 medium (BioWhittaker) and homogenized through a 70 μm nylon sieve. To determine bacterial burden (CFU), cell suspensions were serially diluted onto 7H11 agar plates supplemented with OADC. After 18 days at 37°C, visible colonies were counted and the bacterial load was calculated per organ. For cell culture, erythrocytes were lysed using 0.83% ammonium chloride in water, before lung cells (1 × 106 / ml) and splenocytes (5 × 106 / ml) were restimulated with 20 μg/ml purified-protein derivative (PPD) (SSI, Denmark) and 2 μg/ml anti-CD28 (NIMR; 37.51) for 16 h in 24-well tissue culture plates (final well volume was 1 ml). In the initial experiments using C57BL/6 and C57BL/6 Il10−/− mice, 10 μg/ml PPD alone was used for cell restimulations for 48 h. Supernatants were collected at these timepoints and assessed by ELISA for IFN-γ or IL-6 concentrations using matched antibody pairs (NIMR) (limits of detection 70 pg/ml and 50 pg/ml respectively), and TNF-α or IL-17A using ELISA kits (eBioscience; limit of detection 30 pg/ml for each) following manufacturer’s instructions. Brefeldin A (10 μg/ml; Sigma-Aldrich) was added to the cultures for the last 4 h before the cells were stained for extracellular markers and intracellular cytokines for analysis by flow cytometry.
Flow cytometry
Harvested cells were washed in Dulbecco’s PBS (Gibco), treated with 5 μg/ml anti-FcγRI/FcγRII (anti-CD16/CD32; NIMR; 2.4G2), stained to exclude dead cells using red LIVE/DEAD Fixable Dead Cell Stain Kit (Invitrogen) according to the manufacturer’s instructions, and stained for extracellular markers in PBS on ice. Intracellular staining was carried out using a BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. After staining, samples were fixed with BD Stabilizing Fixative (BD Biosciences) and refrigerated overnight before acquisition on a CyAN ADP analyzer (Dako, Ely, UK) using Summit software (Cytomation). Results were analyzed using FlowJo software (TreeStar, vers. 8.8.6). Cytokine-producing cell numbers and percentages shown in column graphs have had background staining subtracted using identically gated unstimulated populations. The following Abs were used in experiments: γδTCR-FITC (GL3), CD8α-PerCPe710 (53-6.7), Thy1.2-PE-Cy7 (53-2.1), IFN-γ-e450 (XMG1.2) (all purchased from eBioscience); CD4-V500 (RM-45, BD Horizon), CD3-APC (17A2, BD Pharmingen), and IL-17A-PE (TC11-18H10, BD Pharmingen).
Statistical analysis
Data were analyzed as indicated in the figure legends using either one-way ANOVA with Bonferroni’s multiple comparisons test, or two-tailed Student’s t-test using Graphpad Prism software. Differences were considered significant when p < 0.05.
Results
BCG vaccination in the absence of IL-10 enhances IFN-γ and IL-17 responses and increases protection against Mtb challenge
Infection or vaccination with BCG induces IL-10 production in both humans (47, 48) and mice (45, 49), and we have observed that T cells are a major source of IL-10 following BCG vaccination (data not shown). Previous studies by others have shown that IL-10 reduces the cytokine response to systemic BCG infection in vivo, with Il10−/− mice displaying significantly lower BCG bacterial burden and no signs of host-mediated immunopathology (45, 50). In accord with this, recent studies in our laboratory have shown an enhanced and earlier cytokine response in the lung, spleen, and serum of Il10−/− mice following aerogenic Mtb infection compared to WT control mice (36). In light of this work and the work of others (24, 45), we questioned whether BCG vaccination of Il10−/− mice could broadly enhance cytokine responses leading to greater protection against aerogenic Mtb challenge.
C57BL/6 or C57BL/6 Il10−/− mice were vaccinated with BCG and, after 16-weeks, mice were killed and lung and spleen cell suspensions were restimulated ex vivo with Mtb PPD. Levels of IFN-γ, TNF-α, and IL-17A from splenocytes were significantly increased in BCG-vaccinated C57BL/6 Il10−/− mice when compared to vaccinated WT animals (Fig. 1). The same significant increase in the levels of these cytokines from PPD-restimulated splenocytes was also observed 6-weeks following BCG-vaccination of C57BL/6 mice in the presence of anti-IL-10R mAbs, as compared to BCG vaccination in the presence of isotype control mAbs (Supplemental Fig. 1). These cytokines could not be detected from PPD-restimulations of lung cells (data not shown), likely due to the lack of recruitment of T cells to the lung following intradermal BCG vaccination.
FIGURE 1.
C57BL/6 Il10−/− mice have enhanced IFN-γ and IL-17 responses in the spleen after intradermal BCG vaccination. C57BL/6 or C57BL/6 Il10−/− were vaccinated with either 5 × 105 CFU BCG i.d. or PBS i.d. as control. After 16 weeks, splenocytes were isolated and were restimulated with PPD as described in Materials and Methods. The level of production of IFN-γ, TNF-α, and IL-17A were measured from the supernatants of these restimulations by ELISA. ND = below the level of detection. Data shown are mean ± SEM from two combined identical experiments, totaling six mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA.
The increased levels of PPD-induced IFN-γ and TNF-α from BCG-vaccinated Il10−/− mice, both well characterized protective factors in the immune response to Mtb (6, 9, 51), led us to investigate whether these mice would show any increased protection to subsequent Mtb challenge. Six weeks following BCG (or PBS control) vaccination, C57BL/6 or C57BL/6 Il10−/−mice were challenged with low-dose Mtb by aerosol, and at 28- and 56-days post challenge mice were killed and the bacterial loads in the lungs and spleen were determined (Fig. 2). At day 28 post Mtb challenge, BCG-vaccinated Il10−/− mice showed lower bacterial burdens in the lung compared to BCG-vaccinated WT animals (Fig. 2) in line with a previous report (24). There was no difference in Mtb bacterial load between PBS-administered WT and Il10−/− groups at this timepoint. No differences in CFU were observed at day 28 between any of the groups in the spleen, possibly due to low and variable levels of dissemination at this timepoint (Fig. 2). At day 56 post challenge, BCG-vaccinated Il10−/− mice continued to show enhanced bacterial control in the lungs as compared to BCG-vaccinated WT counterparts. As previously reported, the bacterial load was lower in the Il10−/− mice at this timepoint after Mtb challenge independent of BCG vaccination (36). Furthermore, the Mtb bacterial load in the spleen was also significantly lower in BCG-vaccinated Il10−/− mice at day 56 (observed in two separate identical experiments, Supplemental Fig. 2), and showed only a minimal increase from day 28, suggesting greater control of Mtb dissemination in these animals (Fig. 2 and Supplemental Fig. 2).
FIGURE 2.
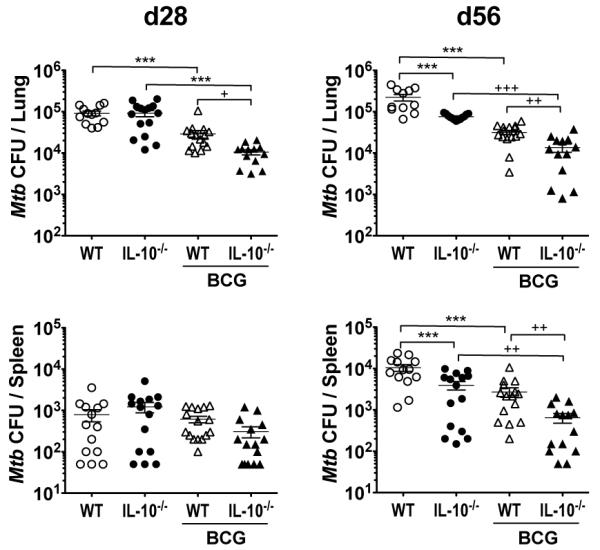
BCG-vaccinated C57BL/6 Il10−/− mice show greater protection to Mtb challenge. C57BL/6 or C57BL/6 Il10−/− mice were vaccinated with either 5 × 105 CFU BCG i.d. or PBS i.d. as control. Mice were challenged by Mtb aerosol 6-weeks after vaccination, and bacterial load per lungs and spleen was determined at 28-days and 56-days post challenge. Data shown are mean ± SEM from two combined identical independent experiments (n = 5-8 per group, per experiment) totaling 12-16 per group. ***p < 0.001 by one-way ANOVA; +p < 0.05, ++p < 0.01, +++p < 0.001 by unpaired Student’s t-test.
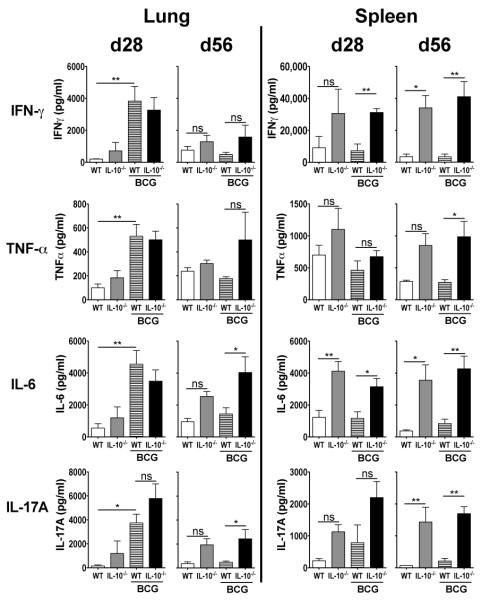
To determine the immune responses underlying the enhanced protection observed, we carried out ex vivo PPD-restimulations on lung and spleen cell suspensions from these mice (Fig. 3). Concentrations of IFN-γ, TNF-α, IL-6, and IL-17A from lung restimulations were significantly enhanced at day 28 post Mtb challenge in WT mice that had been BCG-vaccinated compared to PBS-treated WT animals. No significant differences were observed between BCG-vaccinated WT and BCG-vaccinated Il10−/− mice at this timepoint. By day 56 following Mtb challenge, the concentrations of these cytokines however showed no significant differences between BCG-vaccinated WT and PBS-treated WT mice. Production of TNF-α, IL-6, and IL-17A remained more strongly sustained in the lungs of BCG-vaccinated Il10−/− mice compared to BCG-vaccinated WT animals at day 56 post challenge. Cytokine responses in the spleen were generally greatest in Il10−/− mice post Mtb challenge, regardless of BCG vaccination, and were elevated in comparison to WT animals at both timepoints investigated.
FIGURE 3.
BCG-vaccinated C57BL/6 Il10−/− mice have sustained cytokine levels during Mtb challenge. C57BL/6 or C57BL/6 Il10−/− were vaccinated and Mtb-challenged as described in Fig. 2. Lung cells and splenocytes were isolated at 28- and 56-days post challenge and were restimulated with PPD as described in Materials and Methods. IFN-γ, TNF-α, IL-6, and IL-17A were measured in the supernatants of these restimulations by ELISA. Data shown are mean ± SEM from two combined identical independent experiments (n = 5-8 per group, per experiment) totaling 12-16 per group. ns = not significant. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA.
Taken together, these data confirm that BCG vaccination in the absence of IL-10 increases protection in the lung (24); but extend upon these findings by showing that decreased Mtb bacterial burdens in the lungs are also observed at later timepoints post Mtb challenge, as well as in the spleens, of BCG-vaccinated Il10−/− mice. Furthermore, the enhanced protection seen in these mice is associated with increased PPD-specific cytokine responses in both the lungs and spleen post Mtb challenge. However, from these experiments it is unclear whether these effects are at the level of infection, vaccination, or both.
IL-10R blockade during BCG vaccination alone enhances protection against Mtb in resistant C57BL/6 mice
As Il10−/− mice have previously been shown to have an earlier and enhanced immune response to aerogenic Mtb infection resulting in significantly reduced bacterial burdens (33, 36, 37), we determined whether abrogation of IL-10 signaling during BCG vaccination only would lead to enhanced protection against Mtb challenge. This would show whether IL-10 has a regulatory role specifically at the level of initial BCG vaccination, as has been shown in other immunization models (38-42). To do this we administered anti-IL-10R mAb, or isotype control mAb, to C57BL/6 mice during BCG vaccination only, for 6 weeks, stopping mAb administration for 2 weeks before mice were challenged with Mtb (Fig. 4). Mtb bacterial load was measured at 28- and 63-days post Mtb challenge in lungs and spleens. At day 28 post Mtb challenge, although BCG-vaccinated/isotype-treated mice had significantly greater protection following Mtb challenge compared to unvaccinated/isotype-treated animals, there was no additional protection observed in BCG-vaccinated/anti-IL-10R-treated mice. However by day 63 post Mtb challenge, BCG-vaccinated/anti-IL-10R-treated mice showed a decrease in lung bacterial load over BCG-vaccinated/isotype-treated animals (Fig. 4). This difference was also observed in the spleen at day 63 (although this did not reach statistical significance; p = 0.173). These data suggest that blockade of IL-10R signaling at the time of administering BCG enhances the vaccine-driven protective host response against Mtb infection.
FIGURE 4.
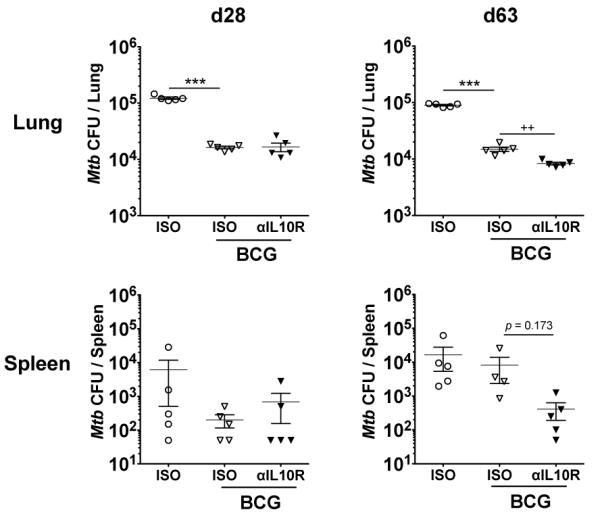
Anti-IL-10R mAb treatment during BCG vaccination increases protection against Mtb in resistant C57BL/6 mice. C57BL/6 mice were treated with 1 mg anti-IL-10R mAb or isotype control mAb i.p. and the next day vaccinated with either 5 × 105 CFU BCG i.d. or PBS i.d. as control. Mice then received 0.35 mg anti-IL-10R mAb or isotype control mAb i.p. weekly following vaccination for 6-weeks. Two weeks after the final weekly mAb administration, mice were challenged by Mtb aerosol, and bacterial load per lungs and per spleen was determined at 28-days and 63-days post challenge. Data shown depict the mean ± SEM from one experiment (n = 5 per group). ***p < 0.001 by one-way ANOVA; ++p < 0.01 by unpaired Student’s t-test.
Anti-IL-10R mAb treatment during BCG vaccination of Mtb-susceptible CBA/J mice significantly enhances protection against aerogenic Mtb challenge
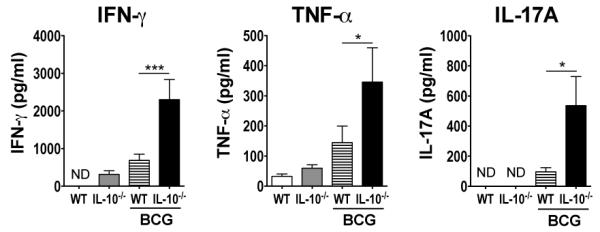
Strains of mice that are more susceptible to Mtb have been argued to better reflect the pathology of human TB than the more commonly used C57BL/6 and BALB/c Mtb-resistant strains (52, 53). The CBA/J mouse strain, alongside DBA/2 and C3H strains, is more susceptible to Mtb infection, with escalating bacterial burden in the lungs until death at around 150-days post aerosol infection (34, 35, 52, 54, 55). To determine whether blockade of IL-10 signaling during BCG vaccination could also induce a greater cytokine response in this susceptible strain, CBA/J mice were vaccinated as before with concomitant administration of anti-IL-10R mAb or isotype control mAb. Seven weeks following the final mAb administration (13 weeks post BCG vaccination) we determined the PPD-specific cytokine response (Fig. 5). BCG vaccination with concomitant blockade of IL-10 signaling induced significantly greater levels of IFN-γ and TNF-α from splenocytes compared to BCG vaccination alone (Fig. 5). Low levels of PPD-specific IL-17A were also observed from these mice, but not from any other group. PPD-specific cytokine production was not detected in lung cell supernatants from these mice (data not shown).
FIGURE 5.
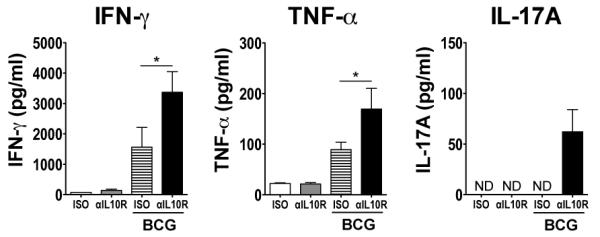
Blockade of IL-10R signaling during BCG vaccination enhances Th1- and Th17-type responses in Mtb-susceptible CBA/J mice. CBA/J mice were treated with 1 mg anti-IL-10R mAb or isotype control mAb i.p. and the next day vaccinated with either 5 × 105 CFU BCG i.d. or PBS i.d. as control. Mice then received 0.35 mg anti-IL-10R mAb or isotype control mAb i.p. weekly following vaccination for 6-weeks. Seven weeks following the last mAb administration (13 weeks post BCG vaccination), splenocytes were isolated and were restimulated with PPD and anti-CD28 as described in Materials and Methods. The level of production of IFN-γ, TNF-α, and IL-17A were measured from the supernatants of these restimulations by ELISA. ND = below the level of detection. Data shown are mean ± SEM from two combined identical experiments, totaling six mice per group. *p < 0.05, **p < 0.01 by one-way ANOVA.
We next investigated whether anti-IL-10R mAb treatment in combination with BCG vaccination would increase protection against Mtb challenge in Mtb-susceptible CBA/J mice (Fig. 6). Strikingly, at day 63 post Mtb challenge, BCG-vaccinated CBA/J mice that had received anti-IL-10R mAb during vaccination had a 10-fold lower bacterial burden in the lungs compared to isotype control mAb-treated BCG-vaccinated animals, extending to a 13-fold difference by day 112 post-challenge (Fig. 6). Whereas the effect of BCG vaccination alone appeared to wane at 112-days post Mtb challenge, this was to a much greater extent than in mice that had been vaccinated with BCG in the presence of anti-IL-10R mAbs. These data suggest that blockade of IL-10R during BCG vaccination results in a sustained protective control of bacterial load, and provides significantly greater protection than BCG alone. The bacterial load in the spleen was similarly reduced at day 112 in BCG-vaccinated/anti-IL10R-treated mice and showed the smallest increase from the spleen bacterial burdens at day 63 (Fig. 6). At both timepoints investigated it was observed that PBS-vaccinated/anti-IL-10R-treated control mice had a small reduction in bacterial load compared to PBS-vaccinated/isotype-treated animals. This was more marked at the earlier timepoint suggesting a low remaining concentration of the anti-IL-10R mAb early during Mtb infection, despite ceasing administration before Mtb challenge (Fig. 6).
FIGURE 6.
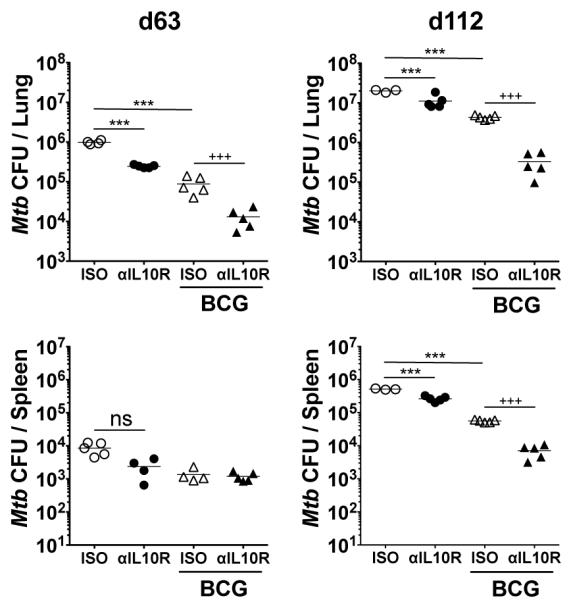
Blockade of IL-10R signaling during BCG vaccination significantly increases protection to Mtb challenge in the susceptible CBA/J strain. CBA/J mice were treated with 1 mg anti-IL-10R mAb or isotype control mAb i.p. and the next day vaccinated with either 5 × 105 CFU BCG i.d. or PBS i.d. as control. Mice then received 0.35 mg anti-IL-10R mAb or isotype control mAb i.p. weekly following vaccination for 6-weeks. One week after the final weekly mAb administration, mice were challenged by Mtb aerosol, and bacterial load per lungs and per spleen was determined at 63-days and 112-days post challenge. Data shown is from one of two independent experiments (n = 5 per group) and depict the mean ± SEM. ns = not significant. ***p < 0.001 by one-way ANOVA; +++p < 0.001 by unpaired Student’s t- test.
To explore potential mechanisms to account for the considerable protection observed by blockade of IL-10R-signaling during BCG vaccination in CBA/J mice, we examined how the immune response was altered during the Mtb challenge of these animals. We were unable to find any significant differences in total numbers of CD4+ T cells, CD8+ T cells, or γδ T cells in the lungs between any of the groups at 28-, 63-, and 112-days post challenge (data not shown). This suggests no clear evidence of earlier migration of lymphoid cells to the lung at these timepoints that could explain the significantly increased protection observed in BCG-vaccinated/anti-IL-10R-treated animals. As vaccine-induced T cells are likely to migrate at earlier timepoints to the lung post Mtb challenge, we investigated the percentages and total numbers of PPD-specific cytokine-producing T cells at day 20 post infection of BCG-vaccinated/anti-IL-10R-treated C57BL/6 mice (Supplemental Fig. 3). At this early timepoint post challenge, we were able to observe increases in the percentages and numbers of IFN-γ-producing CD4+ T cells in mice that had received anti-IL-10R mAb only during BCG vaccination, compared to BCG vaccination alone. We did not observe cytokine-producing CD8+ T cells or innate lymphocytes at day 20 post challenge, nor any cells positive for intracellular IL-17A at this timepoint (Supplemental Fig. 3; and data not shown).
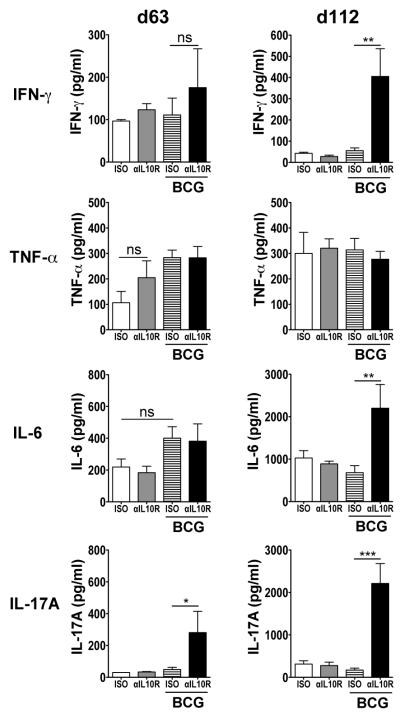
PPD-restimulation of lung cell homogenates from Mtb-challenged BCG-vaccinated/anti-IL-10R-treated CBA/J mice revealed elevated production of IFN-γ at day 112 post challenge, compared to BCG-vaccinated/isotype-treated counterparts (Fig. 7). Levels of IFN-γ at day 112 post challenge in the other groups of mice were however seen to wane to lower levels as compared to day 63, whereas IFN-γ concentrations in BCG/anti-IL-10R-treated mice remained high. Levels of PPD-specific TNF-α in restimulated lung cell suspensions were similar between all the Mtb-challenged groups, at both timepoints investigated. In BCG-vaccinated/anti-IL-10R-treated mice, IL-17A production was enhanced in the lungs at 63-days post challenge, though by day 112, PPD-elicited production of IL-17A and IL-6 was strongly enhanced compared to all other control groups (Fig. 7).
FIGURE 7.
The enhanced protection in anti-IL-10R mAb-treated/BCG vaccinated CBA/J mice is accompanied by enhanced PPD-specific cytokine responses in the lung during Mtb challenge. CBA/J mice were vaccinated with either concomitant anti-IL-10R mAb or isotype control mAb, and Mtb-challenged as previously described for Fig. 6. Lung cells were isolated at 63- and 112-days post challenge and were restimulated with PPD and anti-CD28 as described in Materials and Methods. IFN-γ, TNF-α, IL-6, and IL-17A were measured in the supernatants of these restimulations by ELISA. Data shown are from one of two independent experiments (n = 5 per group) and depict the mean ± SEM. ns = not significant. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA.
Lung cellular sources of cytokine production in Mtb-challenged CBA/J mice after vaccination with BCG in the presence of anti-IL-10R mAb
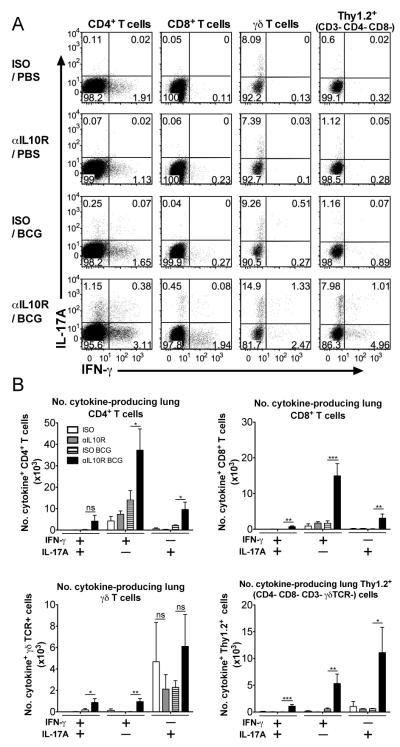
To identify the cellular sources of PPD-specific IFN-γ and IL-17A during Mtb challenge, intracellular cytokine staining was carried out on the PPD-restimulated lung cell cultures for flow cytometric analysis. Live T cell populations were gated as shown (Supplemental Fig. 4A). Analysis of IFN-γ and IL-17A production by lung cells taken from mice at days 63 (data not shown) and 112 post Mtb challenge, revealed that the percentages of IFN-γ-producing CD4+ Th1 and also IL-17A-producing CD4+ Th17 T cells were increased in BCG-vaccinated/anti-IL-10R-treated animals compared to all other groups (Fig. 8A, and Supplemental Fig. 4). These findings also translated to total numbers of cytokine-producing cells (Fig. 8B). Similarly, percentages and numbers of PPD-specific IFN-γ and IL-17-producing CD8+ T cells were significantly increased in this group compared to BCG-vaccinated/isotype-treated mice (Fig. 8, and Supplemental Fig. 4). The majority of IFN-γ+ cells in the lungs of BCG-vaccinated/anti-IL-10R-treated CBA/J mice were CD4+ T cells or CD8+ T cells at day 112 post Mtb challenge. A major source of IL-17A was from γδ T cells during Mtb challenge, regardless of vaccination, as has previously been shown during either Mtb or BCG infections in the lungs of mice (56, 57), though this was not significantly higher in BCG-vaccinated/anti-IL-10R-treated mice as compared with other groups (Fig. 8). However, a high percentage of IL-17A+ cells was seen in a Thy1.2+ CD3−CD4−CD8−γδTCR− innate-like lymphoid population in BCG-vaccinated/anti-IL-10R-treated mice that was greatly enhanced over BCG vaccination alone, where these IL-17A-producing cells were barely detectable (Fig. 8A and Supplemental Fig. 4). This innate lymphoid population also made IFN-γ after Mtb challenge of BCG vaccinated mice in the absence of IL-10R signaling. Blockade of IL-10 signaling during BCG vaccination resulted in small increases in numbers of IFN-γ/IL-17A double-producing CD4+, CD8+, γδ T cells and innate-like Thy1.2+ CD3− cells in the lung on Mtb challenge (Fig. 8B,).
FIGURE 8.
CD4+ and CD8+ T cells, and innate-like Thy1.2+CD3−CD4−CD8− γδTCR−cells, are major sources of IFN-γ and IL-17A during Mtb challenge of BCG-vaccinated/anti-IL10R-treated CBA/J mice. CBA/J mice were BCG-vaccinated with either concomitant anti-IL-10R mAb or isotype control mAb, and Mtb-challenged as described for Fig. 6. Lung cells were isolated at 112-days post challenge and were restimulated with PPD and anti-CD28 as described in Materials and Methods, with brefeldin-A added to the culture for the last 4 h. Intracellular staining was performed to determine the percentages of IFN-γ- and IL-17A-producing lymphoid cells by flow cytometry (A), and the total numbers of cytokine-positive cells in the lung were calculated using the total cell counts acquired from each individual mouse (B). Dot plots shown are concatenated from all individuals per group (A). Data shown is from a representative experiment of two timepoints with similar results (n = 5 per group). ns = not significant. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA.
Discussion
Despite its efficacy in protecting infants from disseminated forms of TB, the only current TB vaccine, BCG, has proved variable for protection against pulmonary disease (3-5). The global burden of TB, and the variability in the current BCG vaccine, highlights the need to gain greater understanding of the complex balance of immune mediators following vaccination, and their correlation with the best levels of protection (3, 5). In this study we asked whether IL-10 action following BCG vaccination limited vaccine efficacy in terms of the T cell responses generated, and most importantly whether this would limit protection against Mtb challenge. We found that blockade of IL-10 signaling during BCG vaccination increased PPD-specific Th1, Th17, and innate lymphoid IFN-γ and IL-17 responses, and that this regime enhanced the protection provided by BCG against Mtb challenge in two mouse strains with different Mtb susceptibility.
Our findings are in line with the role of IL-10 in suppressing vaccination against other pathogens. Blockade of IL-10 signaling during vaccination against L. major infection has similarly been shown to boost Th1 responses, and enhance parasitic control following challenge with Leishmania major promastigotes (39-42). Also, administration of anti-IL-10R mAb during chemotherapy of Schistosoma mansoni infection increases anti-worm Th1 and Th17 responses, correlating with significantly enhanced resistance to re-infection (58). The increased Th1 responses in these studies, and in our own observations following BCG vaccination in the absence of IL-10 signaling, is likely to be due to removal of the regulation of IL-10 on antigen-presenting cells (APCs), including inhibition of IL-12 production and of cell surface molecules involved in antigen presentation (28, 37). In our study, the highest PPD-specific IFN-γ responses were seen to occur in BCG-vaccinated C57BL/6 Il10−/− mice and BCG-vaccinated/anti-IL-10R-treated CBA/J mice; however despite the necessity of IFN-γ production in control of Mtb infection, several studies have demonstrated that the level of IFN-γ alone does not correlate with protection (59-61). Therefore, the high level of protection in these animals is unlikely to be solely explained by increased levels of this cytokine.
The increased Th17 responses we observed may also be partly due to released inhibition of APC function following anti-IL-10R mAb treatment. However, in light of recent data showing that IL-10 can act directly on Th17 cells to regulate their responses (62), and can promote regulation of Th17 cells via effects on regulatory T cells (63), the blockade of direct IL-10R signaling on these cell types may also explain the increased proportion of Th17 cells in BCG-vaccinated/anti-IL-10R-treated mice. In line with this we also observe increased mRNA levels of IL-17A, IL-17F, and IL-22 in the lungs of BCG-vaccinated/anti-IL-10R-treated CBA/J mice during Mtb challenge (data not shown). The presence of IL-17-producing CD4+ T cells in the lung following peptide/adjuvant vaccination has previously been shown to be necessary for the early recruitment of Th1 memory cells to the lung on Mtb challenge (23). Although throughout our study we see a strong correlation between protection and lung IL-17A levels in BCG-vaccinated mice with blockade or absence of IL-10 signaling, a direct contribution of IL-17A to efficient recruitment of Th1 cells and/or the increased protection in this setting remains to be determined.
As well as increased CD4+ Th1 and Th17 cell responses, we also observed increased numbers of IFN-γ- and IL-17A-producing CD8+ T cells, γδ T cells, and an innate-like Thy1.2+CD3− population in the lungs of Mtb-challenged BCG-vaccinated/anti-IL-10R-treated CBA/J mice. A previous study has demonstrated that γδ T cells and a non-CD4+CD8+ population of cells produce IL-17 following primary Mtb infection in vivo (56), though whether the latter IL-17-producing population is similar to the Thy1.2+CD3− IL-17A-producing cells we observe is not known. The Thy1.2+CD3− population we observed may include NK cells, or could include IFN-γ- and IL-17A-producing members of the recently described family of innate lymphoid cells (64, 65). The increased numbers of CD8+ T cells observed to produce IFN-γ and IL-17A may be of advantage to the host given the important ancillary role of these cells in Mtb infection alongside CD4+ T cells (6, 51), and evidence that they may limit Mtb dissemination by occupying the outer lymphocyte infiltrate of the TB granuloma (66).
Irrespective of its source, a major role of IL-17 in many infectious diseases is the promotion of granulocyte accumulation in tissues, including neutrophils (67). The adverse and beneficial roles of this latter cell type in TB are under much scrutiny (68). A recent study has shown an association between IL-17-dependent accumulation of neutrophils and severe lung immunopathology in Mtb-infected mice given subsequent repeated BCG vaccination (25). Although we observed a significantly elevated level of IL-17A in the lungs of Mtb-challenged BCG-vaccinated/anti-IL-10R-treated CBA/J mice, we saw no matching increase in total numbers of lung neutrophils at any of the timepoints investigated (data not shown). In light of recent data showing that the presence of IFN-γ regulates neutrophils during Mtb infection (69), we postulate that the high levels of IFN-γ in these mice control the emergence of adverse IL-17-driven neutrophil-associated lung inflammation.
Perhaps the most striking finding of this study was the significantly enhanced protection seen in CBA/J mice that had received anti-IL-10R blocking mAb in parallel with BCG vaccination. The greater protective effect of this regime in the CBA/J strain as compared to C57BL/6 animals at 63-days post Mtb challenge may reflect their inherent differences in susceptibility to Mtb infection (54). Importantly, BCG-vaccinated/anti-IL-10R-treated susceptible CBA/J mice had sustained control of Mtb infection, as at 112-days post Mtb challenge a 13-fold additional decrease in lung Mtb bacterial burden was observed, compared to BCG-vaccinated/isotype-treated counterparts. We presume the effect of anti-IL-10R mAb in enhancing BCG-mediated protection occurs predominantly during the initial period of vaccination as the last administration of antibody was at least 1-week before Mtb challenge, and the protective effects we have observed occur several weeks post challenge. We cannot however exclude the possibility of remaining anti-IL-10R mAb in the system during Mtb challenge, although this may be beneficial for the host given the negative regulatory effect IL-10 plays during primary Mtb infection (33, 34, 36). The use of Mtb-susceptible mouse strains such as the CBA/J alongside the commonly used Mtb-resistant C57BL/6 and BALB/c strains, is important in the development of new TB vaccines, given that susceptible mouse strains may more accurately depict humans that progress to active TB (52).
An important obstacle in the development of efficient protection in the lung following Mtb infection is the time it takes for T cells to migrate to infected areas where they can help direct macrophage-mediated killing of internalized bacilli via the production of IFN-γ (6, 53). Indeed, it has been demonstrated that the earlier control of a secondary aerogenic Mtb infection in antibiotic-cured ‘Mtb-immunized’ mice, correlates with the earlier migration of Th1 cells to the lung (19). Our previous data show that the enhanced protection of Il10−/− mice following aerogenic Mtb infection also occurs with earlier and enhanced lung cytokine responses (36). In the present study, we observed that BCG-vaccinated/anti-IL-10R-treated C57BL/6 mice have a small but significant increase in IFN-γ+ CD4+ T cells at day 20 post Mtb challenge, when compared to control groups (Supplemental Fig. 3). This may be the result of earlier migration of Mtb-specific CD4+ T cells to the lungs, and may play some part in the increased level of protection to Mtb challenge observed following BCG-vaccination in the absence of IL-10 signaling. More detailed early kinetics experiments of T cell migration during Mtb challenge of BCG-vaccinated/anti-IL-10R-treated mice will shed more light on these dynamics.
In summary, our data shows that inhibition of IL-10 signaling during BCG vaccination increases and balances IFN-γ and IL-17A responses in favor of the host, providing significantly enhanced and sustained protection to aerogenic Mtb challenge over BCG vaccination alone, without inducing overt host pathology. The enhanced protection to Mtb challenge is associated with increased Th1, Th17, and innate lymphoid IFN-γ and IL-17 responses, is sustained during Mtb challenge, and increases protection in both Mtb-susceptible and Mtb-resistant mouse strains. This study gives added insight into the negative regulatory role of IL-10 during vaccination, and suggests that the use of antagonists of IL-10 signaling may be of great benefit as adjuvants in preventive vaccination against TB.
Supplementary Material
Acknowledgements
We thank MRC NIMR Biological and Procedure Services, Safety Services, and the Flow Cytometry Facility at MRC NIMR for technical help and assistance. We also thank DNAX, now Merck, Palo Alto, USA for provision of 1B1.3a and GL113 hybridomas. We thank Leona Gabryšová, Ashleigh Howes, John Ewbank, Finlay McNab and Philippa Stimpson for their feedback on the manuscript. We thank MRC UK for funding this study.
Source of support: This work was supported by the Medical Research Council, United Kingdom (U117565642). Douglas Young is supported by the Medical Research Council, United Kingdom (U117581288).
Abbreviations used in this article
- BCG
Mycobacterium bovis bacillus Calmette-Guérin
- i.d.
intradermal
- IL-10R
IL-10 receptor
- Mtb
Mycobacterium tuberculosis
- NIMR
Medical Research Council National Institute for Medical Research
- PPD
purified protein derivative
- TB
tuberculosis
- WT
wild-type
References
- 1.W.H.O. Global Tuberculosis Control 2011. World Health Organisation; 2011. [Google Scholar]
- 2.Dorman SE, Chaisson RE. From magic bullets back to the magic mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2011;33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 5.Young DB, Perkins MD, Duncan K, Barry CE., 3rd Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 11.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 14.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper AM, Solache A, Khader SA. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol. 2007;19:441–447. doi: 10.1016/j.coi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 17.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung YJ, LaCourse R, Ryan L, North RJ. ‘Immunization’ against airborne tuberculosis by an earlier primary response to a concurrent intravenous infection. Immunology. 2008;124:514–521. doi: 10.1111/j.1365-2567.2007.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci U S A. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 24.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, Khader SA. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, Castro AG. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper AM. Editorial: Be careful what you ask for: is the presence of IL-17 indicative of immunity? J Leukoc Biol. 2011;88:221–223. doi: 10.1189/jlb.0310146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roach DR, Martin E, Bean AG, Rennick DM, Briscoe H, Britton WJ. Endogenous inhibition of antimycobacterial immunity by IL-10 varies between mycobacterial species. Scand J Immunol. 2001;54:163–170. doi: 10.1046/j.1365-3083.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, Cooper AM. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 35.Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, Vesosky B, Turner J. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–5550. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O’Garra A. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40:2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 38.Castro AG, Neighbors M, Hurst SD, Zonin F, Silva RA, Murphy E, Liu YJ, O’Garra A. Anti-interleukin 10 receptor monoclonal antibody is an adjuvant for T helper cell type 1 responses to soluble antigen only in the presence of lipopolysaccharide. J Exp Med. 2000;192:1529–1534. doi: 10.1084/jem.192.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207:1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts MT, Stober CB, McKenzie AN, Blackwell JM. Interleukin-4 (IL-4) and IL-10 collude in vaccine failure for novel exacerbatory antigens in murine Leishmania major infection. Infect Immun. 2005;73:7620–7628. doi: 10.1128/IAI.73.11.7620-7628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabbara KS, Peters NC, Afrin F, Mendez S, Bertholet S, Belkaid Y, Sacks DL. Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection. Infect Immun. 2005;73:4714–4722. doi: 10.1128/IAI.73.8.4714-4722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005;175:2517–2524. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- 43.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva RA, Pais TF, Appelberg R. Blocking the receptor for IL-10 improves antimycobacterial chemotherapy and vaccination. J Immunol. 2001;167:1535–1541. doi: 10.4049/jimmunol.167.3.1535. [DOI] [PubMed] [Google Scholar]
- 45.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, Hanekom WA. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs M, Brown N, Allie N, Gulert R, Ryffel B. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology. 2000;100:494–501. doi: 10.1046/j.1365-2567.2000.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 52.Apt A, Kramnik I. Man and mouse TB: contradictions and solutions. Tuberculosis (Edinb) 2009;89:195–198. doi: 10.1016/j.tube.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 54.Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marquis JF, Nantel A, LaCourse R, Ryan L, North RJ, Gros P. Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in mice. Infect Immun. 2008;76:78–88. doi: 10.1128/IAI.00369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 58.Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 2011;7:e1002171. doi: 10.1371/journal.ppat.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SH. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, Nouze C, Ladant D, Cole ST, Sebo P, Leclerc C. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74:2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 62.Huber S, Gagliani N, Esplugues E, O’Connor W, Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 65.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:1722–1728. doi: 10.1128/IAI.69.3.1722-1728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 68.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2011;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.