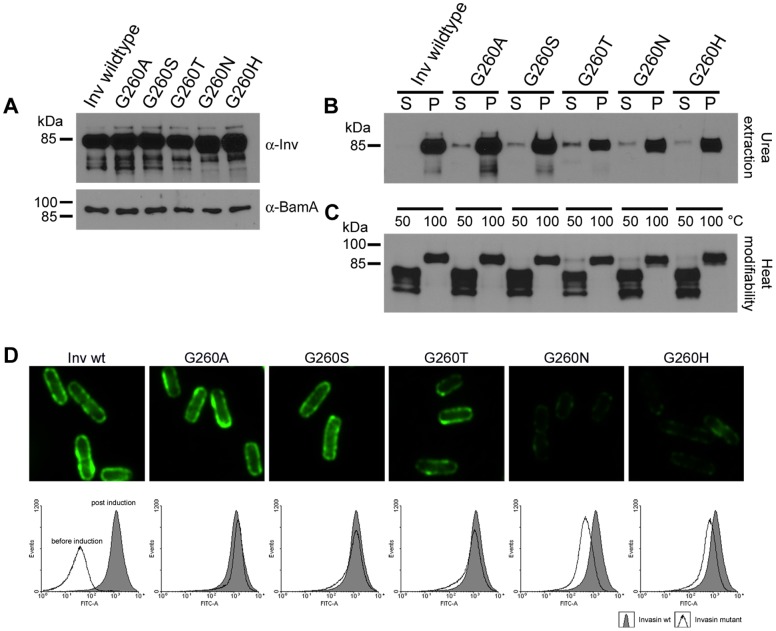
Figure 7. Substitution of a conserved glycine residue leads to impaired autotransport.
A conserved glycine residue (G260) facing the pore lumen of the N-terminal beta-barrel was replaced by amino acids with increasing side chain size (A<S<T<N<H). (A) Production of the invasin variants was analysed by Western blot. All invasin variants are produced in comparable amounts and copurify with the membrane fraction. To demonstrate equal loading the samples were also probed with an antibody directed against BamA. (B) Stable integration of the invasin variants was verified by urea extraction of outer membrane preparations. Only residual amounts of protein can be found in the supernatant whereas the major fraction is stably associated with the outer membrane (S = supernatant, P = pellet fraction). (C) Heat modifiability of all samples indicates proper folding of the beta-barrels. (D) Autotransporter function of the beta-barrels was tested by measuring the outer membrane localisation of effector domains by immunofluorescence and flow cytometry. Histograms show overlays of invasin wild-type (grey filling) and the respective invasin G260 mutants (no filling).