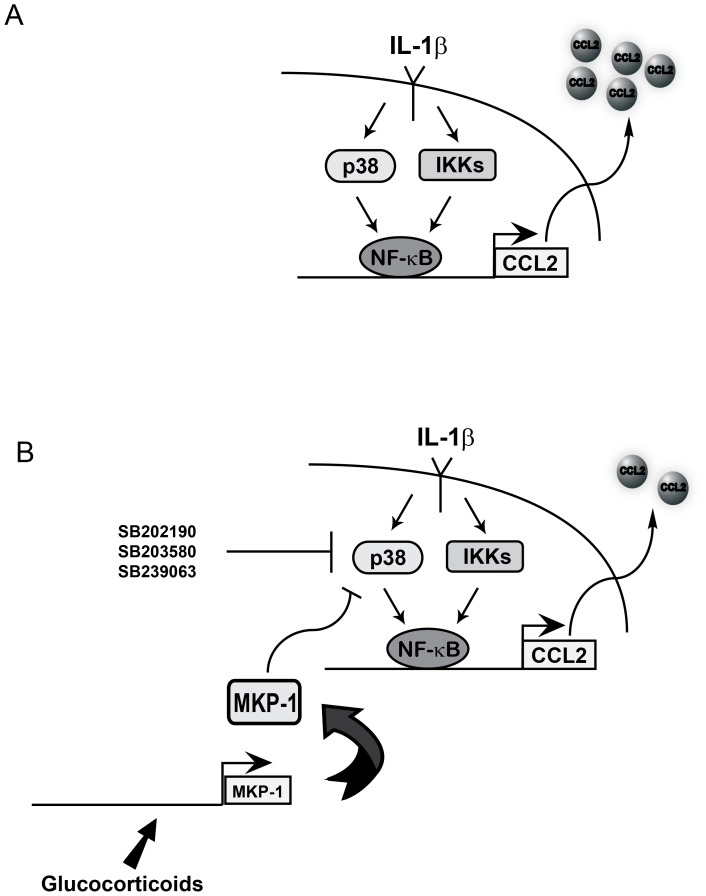
Figure 10. Pharmacological or phosphatase-mediated inhibition of IL-1β-stimulated p38 MAPK phosphorylation decreases synthesis and secretion of CCL2.
A. IL-1β induces release of NF-κB subunits from regulatory proteins by activating the IKKs. In addition, IL-1β promotes phosphorylation of p38 MAPK, increases CCL2 transcription using the NF-κB pathway, culminating with secretion of CCL2 protein. B. The activation of p38 by IL-1β is critical for induction of CCL2 gene expression and secretion of CCL2 protein. Inhibiting p38 MAPK activity, either through the use of pyridinyl-imidazole based inhibitors or via MKP-1 mediated dephosphorylation, impairs synthesis and secretion of CCL2.