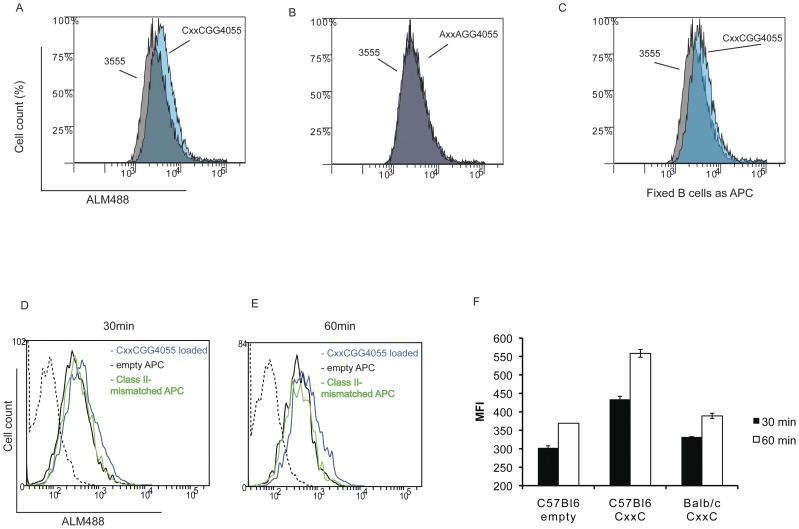
Figure 4. CD4+ T Cell surface thiol level is increased when exposed to CxxC containing peptides.
Splenic B cells were purified from C57BL6 mice by depletion, loaded with 50 µM of peptide MOG35–55, CxxCGG40–55 or loss of function AxxAGG40–55, and added to a Th17 cell line specific for MOG 35–55 (ratio APC/T:1/1). After 1 h, cells were washed and stained with Alexa-maleimide 488 (ALM488) and for CD3 and Ia/Ie molecules and analysed by flow cytometry. Blue histograms show ALM488 staining on T cells (CD3 positive/Ia-Ie negative cells) incubated with CxxCGG40–55 (A) or AxxAGG40–55 (B) loaded B cells in overlay with staining on cells incubated with MOG35–55 loaded B cells (grey histograms). (C), same as in (A), but B cells were fixed with glutaraldehyde after loading with CxxCGG40–55. Data representative of three experiments. (D,E) APC (T cell depleted splenocytes) were loaded with 50 µM peptide CxxCGG40–55 (blue line histogram) or kept unloaded (black line histogram) for 2 h at 37°C. After extensive washes, they were added to the same Th17 cells as described before. Histograms show ALM488 staining of CD3 positive/Ia-Ie negative cells after 30 min (D) or 60 min (E) of co-culture. Class II –mismatched APC (BALB/c) loaded with 50 µM peptide CxxCGG40–55 were used as non-specific binding control (green histogram). Dotted histogram is for background fluorescence of CD4+ T cells. Median fluorescence intensity (MFI) obtained for the two time points (D,E) are shown in (F). Representative of two experiments.