Abstract
Neurogranin (Ng), a brain-specific calmodulin-binding protein, is expressed highly in hippocampus, and is important for cognitive function. Deletion of the Ng gene from mice caused attenuation of signal reaction cascade in hippocampus, impairments in learning and memory and high frequency stimulation-induced long-term potentiation. Environmental enrichment alone failed to improve cognitive function. In the present study, behavioral testing revealed that Ng knockout mice were both hyperactive and socially withdrawn. Methylphenidate (MPH) was given to mice while they were also kept under an enrichment condition. MPH treatment reduced the hyperactivity of Ng knockout mice tested in both the open field and forced swim chamber. MPH improved their social abilities such that mice recognized and interacted better with novel subjects. The cognitive memories of MPH-treated mutants were improved in both water maze and contextual fear conditioning tests. High frequency stimulation-induced long-term potentiation of Ng knockout mice was also improved by MPH. The present treatment regimen, however, did not fully reverse the deficits of the mutant mice. In contrast, MPH exerted only a minimal effect on the wild type mice. At the cellular level, MPH increased the number of glial fibrillary acidic protein-positive cells in hippocampus, particularly within the dentate gyrus of Ng knockout mice. Therefore it will be of interest to determine the nature of MPH-mediated astrocyte activation and how it may modulate behavior in future studies. Taken together these Ng knockout mice may be useful for the development of better drug treatment to improve cognitive and behavioral impairments.
Keywords: Neurogranin knockout mice, cognitive deficit, methylphenidate, environmental enrichment
Introduction
Neurogranin (Ng) is a brain-specific, apo-calmodulin (CaM)-binding, and PKC-specific substrate protein. It is expressed prominently in hippocampus, neocortex, and amygdala, brain regions important for learning and memory. Ng has been considered a CaM buffer (Gerendasy & Sutcliffe 1997; Huang et al. 2000), and CaM was identified as Ng’s sole binding partner in the brain (Prichard et al. 1999). Unlike Ca2+/CaM-dependent enzymes, affinity between CaM and Ng is weakened in stimulated neurons when intracellular [Ca2+] levels are elevated. Ng is also a potent antioxidant protein (Sheu et al. 1996) and it is readily oxidized in the presence of nitric oxide donor or following NMDA receptor stimulation (Li et al. 1999). It is postulated that covalent modifications of Ng by oxidation and phosphorylation, both attenuate its affinity for CaM and prolong the availability of Ca2+/CaM for the activation of Ca2+/CaM-dependent enzymes. Thus, Ng was placed at the top tier of regulatory mechanism for learning and memory (Pak et al. 2000). Indeed, deletion of the Ng gene resulted in deficits in both cognitive functions as well as hippocampal long-term potentiation (LTP) measured in vitro (Huang et al. 2004; Miyakawa et al. 2001; Pak et al. 2000). Compared to wild type (WT) mice, Ng knockout (NgKO) mice not only exhibit learning disabilities, they are also constantly in motion.
Studies on children with Jacobsen Syndrome (chromosome 11q terminal deletion disorder) showed that the cognitive impairments of those inflicted children were variable and dependent on the size of gene deletion (Coldren et al. 2009; Grossfeld et al. 2004). Particularly, deletions of BSX and Ng genes in two separate loci of chromosome 11q have been implicated in the deficit of cognitive function. Most Jacobsen Syndrome children also exhibit a characteristic neuropsychiatric profile of hyperactive and inattentive behavior, often described as attention deficit hyperactivity disorder (ADHD). Many rodent models, including the spontaneously hypertensive rat (Pires et al. 2009; Siesser et al. 2006) and NK1 receptor gene knockout mice (Yan et al. 2010), show ADHD-like symptoms and have been considered as ADHD models when validated by beneficial responses to methylphenidate (MPH), a drug commonly used to treat ADHD children. However, these models lack correlation to the candidate gene study (Kebir et al. 2009).
In the present study, we subjected the Ng KO mice to a battery of behavioral tests to show their abnormal behaviors and cognitive deficits. We also followed their improvement in spatial and fear memory and other behavioral abnormalities following treatment with MPH. Because children harboring the 11q-terminal deletion that encompasses Ng showed cognitive impairment and ADHD-like behaviors, we hypothesized that Ng mutant mice may also exhibit ADHD-like abnormalities and importantly that MPH can alleviate some of these symptoms. These mutant mice therefore may be useful for future development of better therapies for Jacobsen Syndrome children. Initially, we carried out the experiments with regular cage-reared mice and found that MPH affected little of these NgKO mice. Subsequently, experiments were conducted with enrichment cage-reared mice, and we were able to show positive effect of MPH on these mice.
Materials and Methods
Mice and drug treatment
Both WT and NgKO mice were bred in-house by heterozygous mice mating and were colonies of cell line 581 after 12 times back-crossing with C57BL/6J from the original 129X1/SvJ and C57BL/6J mixed background. Animal handling and experimental protocols were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Animals were maintained on a 12h light/dark cycle (6 to 6), and behavioral tests were conducted during the light cycle. Mice used were all female and at one to two weeks post weaning they were transferred to the enrichment cages (48 cm length × 26 cm width × 16 cm height, 6–8 mice/cage), which were equipped with toys and exercise wheels as described previously (Huang et al. 2006). Mice were housed in this enrichment condition at least one week before drug treatment, which was delivered daily via i.p. injection [0.1 ml of either saline or MPH in saline (10mg/kg)]. Drug treatments and environmental enrichment (EE) were continued for 3 weeks before starting and continued throughout both the behavioral testing period, and later electrophysiological experiments. Drug injections were carried out at 9–9:15 AM and behavioral test started 30–45 min later, as was the sacrifice of animal for electrophysiological study.
Behavioral testing
For each experiment, approximately 12–15 WT and KO mice each were randomly divided into saline control and drug treatment groups. Different genotypes were mix caged and all tests were carried out blind. The test was started after 3 weeks of EE and MPH treatment. The test started first with open-field activity measurement (Satoh et al. 2007). Briefly, activity was monitored for 5 min in a Plexiglas enclosed box (42cm × 42cm × 30 cm high by AccuScan Instruments) equipped with DigiPlo software to measure the movement/resting and distance travelled at the marginal and center zone (inside 30 cm square) of the box. On the same day, mice were also tested on an elevated plus maze (San Diego Instruments) for 4 min (Santarelli et al. 2001). The movement and time spent in the open/closed arms were tracked by ceiling-mounted video camera, together with entries were calculated by Videomex program of Columbus Instruments. For the associated learning ability, a contextual fear conditioning protocol (Schafe et al. 1999) was carried out on the 2nd day. Mice were trained individually in a conditioning context for 2 min followed by a foot shock at 0.5 mA that lasted 2 s, and were removed from the shock box 3–5 s later (PACS-30 Columbus Instruments). Twenty four hours later (the 3rd day) the mice were placed back into the same conditioning context and their fear memories were recorded/scored manually as duration of freezing in the 3 min period without further shocking. Afterward, on the same day the mice were subjected to the forced swim test (Overstreet et al. 2005).The mice were placed in a glass cylinder (30 cm high × 20 cm diameter) filled with room temperature water to a depth of 20 cm for 7 min. The duration of time the mouse stopped struggling and remained floating was considered immobile and was recorded. Discriminatory social interaction test was carried out on the 4th day with a modification of protocol described by (Macbeth et al. 2009) in an arena of 15 × 20 in white Teflon enclosure. Briefly, the test consisted of two 5-minute sessions that were 5 min apart. The mouse was placed between two juvenile mice separately confined in upside down stainless wired pencil cups (A and B) kept 4 inches apart. The juvenile in B cup was replaced anew for the second session. In both sessions the movement of the mouse was tracked by ceiling-mounted video camera and its contact with the juvenile mouse in either A or B cup by attaching the nose to the cup was also recorded as interaction.
The following week the mice were subjected to a Morris water maze test (Morris et al. 1982). The pool (105cm in diameter) was filled with room temperature water made opaque with non-toxic paint. On two sides of the wall there were cut-out shapes to serve as landmarks for navigation. For the hidden platform version of the test, mice were trained for 4 days consecutively with 3 blocks of training per day and 3 trials per block where mice were placed against the wall in different quadrants of the pool except the one with the platform. The rest period between blocks was typically greater than two hours. On the first day, before each trial of all 3 blocks, the mouse was placed on the hidden platform for 10s before each trial swim to make it aware of the hidden escape platform which was invisible to them (1–2 cm below the water surface). On the 2nd day this practice was carried out only for the first trial of each block, on the 3rd day only for the first trial of the first block, and none for the 4th day. After each swim, the mouse was pat-dried with cloth, and heating-pad treated after the block (3 swims) to dry the fur before returning to the cage. In each trial, time required for the mouse to reach the target platform (or 1 min maximum) and the actual swimming route were recorded with the ceiling-mounted video and analyzed by Videomex program of Columbus Instrument. On the 5th day a probe test without the platform was administered.
Electrophysiology
Following the conclusion of behavioral tests, the mice were used for electrophysiological experiments, during which time they continued to be housed in the enriched environment and to receive MPH treatment, except for the saline injection for the control group of animals, which was omitted. Mice were sacrificed two per day, and the left side of the hippocampus used for recordings. The corresponding right side of the hippocampus was kept at −80° for later protein expression analysis. Electrophysiological recordings were carried out as previously described (Huang et al. 2007) at 30°C. Briefly, transverse hippocampal slices (400 µm) were prepared and kept in oxygenated aCSF for at least 90 min before using. Slices were submerged in a recording chamber and superfused with oxygenated aCSF at a flow rate of 2 ml/min. Glass electrodes were used both for the stimulation of Schaffer collateral/commissural fibers and the recording of field EPSP (fEPSP) in the stratum radiatum of the CA1 region. Responses were calculated as the change in the slope of fEPSP. After maintaining a stable baseline for at least 20 min at a stimulus current intensity that elicited 1/3 of the maximum response, the slice was subjected to high frequency stimulation at 100Hz for 1 sec, and fEPSPs continued to be recorded for at least the next 60 min. Signals were amplified by an Axon MultiClamp 700B amplifier and digitized by CED Power 1401 (Cambridge Electronic Design). The degree of potentiation was analyzed using Signal 4 (CED) software. The degree of potentiation by high frequency stimulation was expressed as percentage of initial baseline fEPSP. For comparison of responses among different groups the last 10-min blocks of recordings were averaged and analyzed.
Immunoblot analysis
The right side of hippocampus was homogenized in 500 µl of buffer containing 50 mM Tris/Cl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 2 mM DTT, 50 mM KF, 50 µM AEBSF, 5 µg/ml leupeptin, 50 nM okadeic acid, and 1% SDS. Protein concentration was determined by BCA reagent and 30 µg protein was loaded per lane (4 mm wide by 1.5 mm thick) for SDS-PAGE in an 8–16% gradient gel containing 0.1% SDS. After electrophoresis, proteins were transferred onto nitrocellulose membrane. Each membrane was cut into three segments guided by the pre-stained molecular weight standards. The top segment of the membrane was used for blot analysis of GluR1, PKC α and ε, PSD95, and spinophilin; the middle segment was used for p42/p44 MAP kinases, GFAP, αCaMKII, synaptophysin and actin; and the bottom segment for Ng and CaM. Routinely, using the same segment of the membrane, blotting could be analyzed consecutively one antibody after the other with stripping in between. Stripping was carried out by incubating the membrane at 55°C for 30 min in a buffer of 62.5 mM Tris/Cl, pH 7.5, containing 100 mM 2-mercaptoethanol and 2% SDS. Also, each gel contained 4–5 samples from each of control and MPH-treated samples of both WT and KO genotypes for direct comparison among groups of sample. Antibodies used for blotting, (except our original Ab #270 and #3615 (Pak et al. 2000) for Ng and phospho-Ng, respectively,) were all from Cell Signaling Technology and Millipore (Upstate). After the primary and secondary immunoreactions, ECL reagent was used to reveal the protein bands in the linear range of exposure for each antibody. Using Kodak Image Station analysis software, relative intensities of protein bands were scanned and normalized to the intensity of actin band before comparing to those of WT control from the same blot, which was set at 100%.
Immunohistochemistry and confocal image analysis
Following behavioral testing, 4 mice each from control and drug-treated WT and NgKO were used for immunohistochemical analysis. Dissection and preparation of hippocampal slices were identical to that described for electrophysiological experiments. Slices (400 µM) were fixed in 4% paraformaldehyde for at least overnight and those from the mid-location of hippocampus (3–4.2 mm from the ventral end) were further sectioned into 50 µm thickness for the free-float staining. Sections from all groups of animal were processed and stained under identical conditions. For the staining, subsections were treated sequentially with PBS containing 0.5% NP-40 and 3% H2O2 each for a minimum of 15 min and blocked with 2.5% horse serum, and then incubated with mouse anti-GFAP antibody (Cell Signaling, 1/500) or mouse anti-CaM antibody (Zymed, 1/500) in PBS containing 0.025% horse serum and 0.1% thimerosal overnight at room temperature. Immunoreactivity was revealed by incubation of the tissue with ImmPRESS peroxidase-conjugated anti-mouse IgG for 4 h and then with freshly prepared Alexa 594-tyramine fluorescence dye/H2O2 solution at room temperature for 10 min. The reaction was terminated with 10 mM HCl for 30 min. In between incubations, tissue was washed 10 min each with TTBS (20 mM Tris-Cl, pH 7.5, containing 0.5 M NaCl and 0.05% Tween-20) once and PBS twice. Stained sections were blanketed with Vectashield containing DAPI, and cover slipped. Tissue sections incubated either without the first or second antibody exhibited only background staining. Stained tissue sections were examined in a Zeiss LSM 510 inverted microscope with a pin hole setting for red channel (543 nm) at 1 airy unit and the images were captured in 8-bit mode at a high resolution.
For analyzing GFAP-positive profiles, the area of hilus at the dentate gyrus or stratum radiatum at the CA1 field on images captured by a 20× objective was cropped and counted with particle analyzing program of Image J software. The counting was defined for particles greater than 30 µm2 and at 0–1.0 circularity setting that would include non-circular particles. The 20× images analyzed (as shown in Fig. 7) covered the whole area of hilus or the central area of stratum radiatum of CA1 without overlapping with subiculum on one side and CA1/CA2 transition on the other. The counting of such cropped area (roughly 0.1mm2 in both regions) was normalized to unit area for direct comparison. At least two hippocampal sections from each animal (from the mid-location of hippocampus bearing good structural integrity) were analyzed for the GFAP-positive profiles. For analysis of the relative staining intensity of CaM for CA1 pyramidal neurons or granule cells of dentate gyrus, a set of tissue sections containing one of each experimental group of animal (also from mid-hippocampal location) were processed simultaneously and their confocal images were captured with the same gain settings for direct comparison. Mean fluorescence intensity of an area (40 × 40 µm, picked randomly) containing 8–10 neurons was measured by Image J software and about 2–3 non-overlapping areas were sampled from each image as captured by a 40× objective lens. The relative intensity of each area was normalized against the wild type control of the same set, which was set as 100%.
Figure 7.
Immunohistochemical staining and the quantification of GFAP and CaM expression. (A) Representative staining profiles for GFAP and CaM. Images of comparable hippocampal locations from the groups of mice were analyzed. For GFAP, A to D showed the staining at dentate gyrus and E to H at CA1. As antibodies against GFAP labeled astrocytes and not neuron, region shown was image of GFAP (red) merged with blue DAPI nuclear staining. For CaM, I to L showed the labeled pyramidal cell layer of CA1. The antibodies also labeled the granule cell layer of dentate gyrus (not shown). There was much less staining of CaM at apical dendrites than cell layer of CA1. (B) The bar graph summarized the GFAP-positive profiles of the whole hilus area at dentate gyrus and strata radiatum of CA1 per unit area of 0.1 mm2. There were 4 animals in each group and two to three sections of each hippocampus were being counted, n=8 to 9. For CaM, two sections of each hippocampus were being compared, and two to three areas of pyramidal cell layer of CA1 of each sections were being compared, n= 16 to 17. Scale bar: 50 µm.
Statistical analysis
The above described drug treatment experiments under EE were repeated five times and results of the same treated groups of each test were combined and expressed as mean ± SEM. Statistical analyses (using Sigma Stat 3.0 and SYSTAT 11) were made either by one-way or two-way ANOVA analyzing genotype × treatment interaction. For the swimming progress curve, data were analyzed with repeated measures ANOVA. The Holm-Sidak method was used for the post hoc pairwise comparisons to identify the significant differences between two groups. In all cases, p<0.05 was considered significant. All p values shown in the figures were post hoc pair comparisons after ANOVA.
Results
Effect of MPH treatment on Ng mutant mice behavior under enrichment conditions
Because MPH treatment on regular cage-reared mice did not yield any positive effects, all the experiments presented herein were performed under EE condition.
Open field activity
It was readily discernible that NgKO mice were much more active than WT mice (Fig. 1). NgKO mice traveled more than twice the distance than the WT, and were in motion nearly twice the amount of time as WT. In a 5 min-period, KO mice rested only slightly more than the time they moved; whereas WT mice rested almost three times more than they moved. Examining closely the route traveled, it was apparent that NgKO mice traveled mostly around the marginal perimeter of the box. In fact, the total extra distance they traveled was around the edge of the box, while WT mice spent significantly more time in the central area. Importantly, with MPH treatment, NgKO mice showed a significant reduction in the marginal distance they traveled (reduced 589cm), which largely represented the reduction of total distance (reduced 511cm); whereas distance changes in the central area of the box were only minimally affected. Judging from the fact that there were no significant changes between the move/rest times, reduction in the distance traveled could mean that these drug-treated mutant mice moved at a slower speed. Surprisingly, the drug treatment did not change much of the measurements of the WT mice. Statistical analysis showed: for total distance, effect of genotype: F(1,83)=77.611, p<0.001; effect of treatment: F(1,83)=3.658, p=0.059; and genotype × treatment interaction: F(1,83)=5.793, p=0.018. Marginal distance, effect of genotype: F(1,83)=78.615, p<0.001; effect of treatment: F(1,83)=2,168, p=0.145; and genotype × treatment interaction: F(1,83)=3.938, p=0.051. Analysis of move time, effect of genotype: F(1,83)=51.738, p<0.001; effect of treatment: F(1,83)=0.0293, p=0.864; and interaction: F(1,83)=1.653, p=0.202. Central time, effect of genotype: F(1,83)=23.745, p<0.001; effect of treatment: F(1,83)=0.025, p=0.876; and interaction: F(1,83)=1.226, p=0.271.
Figure 1.
Hyperactive behavior of NgKO mice. Open field activity of the animal was recorded for 5 min each; the inside 30 cm square of the box was considered as central area and the outer rim with 6 cm from the wall was marginal area. (A) Distances traveled. Total distances equal to the sums of the marginal and central ones. (B) Move/rest and central/marginal times. The sum of move/rest or central/marginal time was 300 sec. Holm-Sidak’s post hoc pairwise comparisons are shown in the figure. MPH didn’t change significantly any of Wt’s travel distance or any proportion of time they stayed moving or resting, at marginal or central area.
Elevated plus maze
On the elevated plus maze, all groups showed preferences for closed arm than open arm, F(7,220)=29.225, p<0.001. The mutant mice seemed to venture often into the open arm and look down from the edge of the arm. In fact, the total percentage of time that the mutant mice stayed in the open-arm was not significantly more than that of WT mice (Fig. 2A). Probably the mutant mice were more active and have made many more entries in and out of the arms (Fig. 2B and 2C) visibly showing their restless and aimless roaming of the maze. Speeds in either the open or closed arm by one-way ANOVA were not significantly different among the groups, but numbers of entries to either the open or closed arm were significantly different. One- way ANOVA between groups, for open arm: F(3,110)=6.170, p<0.001 and closed arm: F(3,113)=4.749, p=0.004. Two-way ANOVA for the open arm, effect of genotype: F(1,110)=16.686, p<0.001; effect of treatment: F(1,110)=0.511, p=0.476; and interaction: F(1,110)=1.977, p=0.162; and for the closed arm: effect of genotype: F(1,113)=13.951, p<0.001; effect of treatment: F(1,113)=0.06, p=0.807; and interaction: F(1,113)=0.0008, p=0.976. Thus, mutant mice, both treated and untreated, made more entries into the open and the closed arms than the WT mice. MPH treatment did not significantly increase the duration of time, speed of traveling, or number of entries either in WT or mutant mice in the open or closed arm.
Figure 2.
Elevated plus maze, forced swim, and contextual fear conditioning test also showed the restlessness and impaired fear memory of the mutant mice. On elevated plus maze (A), % of time the mice spent visiting and (B), the speed of their traveling in either the open or closed arm were not significantly different among the groups. However, mutant mice made more entries (C) into either the open or closed arm. MPH treatment did not significantly modify any of these profiles. (D) Forced swim test, NgKO mice struggled significantly longer than Wt (p<0.001). MPH did not alter any property of the Wt mice, but caused the mutant mice stop struggling significantly sooner than the untreated mice (p<0.01). (E) In contextual fear conditioning, the KO mice froze significantly less than the Wt (p<0.001), and MPH treatment improved their fear memory significantly against the untreated mice (p<0.001).
Forced swim test
When placed into the small water tank, the mutant mice struggled significantly longer than the WT mice, whose immobile time was 3.5× more than that of the KO mice (Fig. 2D). One-way ANOVA revealed F(3,82)=35.599, p<0.001. MPH did not affect the immobile time of WT mice, but it shortened struggling time of NgKO mice significantly who stayed immobile longer. Two-way ANOVA for the % immobile time showed F(1,81)=108.578, p<0.001 as affected by genotype, F(1,81)=4.064, p=0.047 as affected by treatment, and F(1,81)=3.480, p=0.066 through genotype × treatment interaction.
Contextual fear conditioning
Immediately after the exploration period, the mutant mice appeared to respond to the foot shock with the same intensity as WT. All mice jumped up, squeaked, and became frozen, noticeably showing discomfort. However, if they were removed from the box 3–5 s after shocking and were returned to the same shocking box 24 hours later, most of the WT mice immediately froze and only after 1–2 min would they begin to explore slowly. In contrast most of the mutant mice appeared not to remember the previous aversive experiences and immediately wandered around only with occasional hesitation (Fig. 2E). In a 3-min period, the freezing time of the mutant mice was significantly lower than that of the WT. One-way ANOVA showed differences between groups: F(3,80)=26.948, p<0.001. Regarding genotype, two-way ANOVA revealed F(1,80)=65.098, p<0.001, F(1,81)=1.680, p=0.199 regarding treatment, and F(1,80)=13.085, p<0.001 through genotype × treatment interaction. MPH-treated mutant mice froze almost three times more than the untreated mice. Interestingly, the drug invariably affected the WT mice and only slightly decreased their freezing time.
Discriminatory social interaction
The NgKO mice, besides from being hyperactive and always running around in the cage, also appeared not to pay any attention to their cage mates. They rarely displayed close-following or sniffing of each other when introduced, however they were not any more aggressive in behavior than the WT. In previous EE experiment (Huang et al. 2006) social interaction traits of these mutant mice were not probed, and it was unclear whether EE might have improved their social interaction skill compared to regular home cage-reared mutant mice. The present experiment under enriched conditions revealed that the mutant mice interacted significantly less than the WT control, and the differences were evident in both the first and the second session of encounters (Fig. 3A). One-way ANOVA showed F(3,72)=3.570, p=0.018. The drug treatment increased somewhat the social interaction times of these NgKO mice. Though not statistically significant in the total visit times, the treated mutant mice could differentiate the novel from the familiar mouse during the second session of encounter when a novel subject was introduced (Fig. 3B). One-way ANOVA for the differences between groups revealed F(7,133)=7.153, p<0.001. Thus, for the treated mutant mice, the ratio of visit times of novel (B mouse) over familiar mouse (A mouse) at the second session was significantly increased (p<0.01) over either the first session or either sessions of the untreated mutant mice. Two-way ANOVA for KO mice, effect of treatment: F(1,33)=10.256, p=0.003; effect of session: F(1,33)=9.777, p=0.004; and the interaction: F(1,33)=2.418, p=0.129. For the WT mice, either treated or untreated, the preference to interact with the novel mouse in the second session of meeting greatly exceeded that of the first session, F(3,66)=8,924, p<0.001. However, the increase in the MPH-treated WT mice was not significantly different from that of the control mice.
Figure 3.
NgKO mice are impaired in social interaction. (A) The % interaction time at the 1st and the 2nd sessions. At the 2nd session all groups showed slightly lower interaction time than that of the 1st session. The interaction time of NgKO was lower than that of Wt (p<0.05) in either control or treated group. (B) The ratio of visit time of B/A mouse. The ratio represents the preference of interaction with novel B mouse. MPH-treated mutant exhibited significantly more interaction with the novel B mouse at the 2nd session which was also significantly improved than the control mutant mice.
Morris Water Maze
Mice were subjected to the Morris Water Maze test to assess their hippocampal-dependent spatial memory. During the daily orientation it was clear that the KO mice were very different from the WT mice in that the latter would securely sit on the platform while the former would resist being placed on and jump off it. Even after hitting the goal platform, the KO mice would continue to swim in total ignorance of the platform. Fig. 4A shows the swimming progress curve during the 4 days, 12 blocks of training. One way repeated measures ANOVA revealed that all groups learned, since there was a significant decrease in the escape latencies, although WT learned faster than KO [WT control: F(11,305)=8.265, p<0.0001; WT+MPH: F(11,319)=9.552, p<0.0001; KO control: F(11, 318)=2.170, p=0.016; KO+MPH: F(11,297)=6.385, p<0.001]. Comparing learning curves between groups failed to distinguish either MPH-treated WT or KO from their respective control groups, but showed significant differences between WT vs. KO in both controls and treated groups [control: F(1,53)=28.420, p<0.0001, treated: F(1,56)=27.911, p<0.0001]. Alternatively analysis with repeated measures ANOVA with block as within subject revealed F(11,1188)=15.730, p<0.0001; using block × genotype or block × treatment as within subject did not yield significant p value; but with block × genotype × treatment as factors did, F(11,1188)=2.292, p=0.009. There was, however, a significant effect of MPH on KO mice in the last day (or blocks 10–12) of training between subject: F(1,56)=4.266, p<0.044.
Figure 4.
NgKO mice are restless and clueless swimmers in Morris water maze tasks. (A) The progress curve of escape latencies during the 4 days 12 blocks of training. Mutant mice learned much slower than the Wt mice. In either genotype, MPH did not alter their learning capabilities except in the last three blocks where the treated mutant mice, but not the other groups, continued to shorten their escape latencies. (B) Probe tests. Quadrant 3 (Qd3) was the target quadrant in previous training. During probe test, both control and treated Wt mice spent higher than 25% of swim time in Qd3 indicating skill learned but not the mutant mice. (C) Degree of thigmotaxis. NgKO mice showed strong thigmotaxis throughout all 12 blocks of training. Wt showed it only in the early blocks of training. As their latencies shortened along the blocks so are their degrees of thigmotaxis. (D) Examples of swimming path. Numbers at the right side bottom corner of the track were degree of thigmotaxis. Left panel, tracks 1–7 (KO having 60 sec latency) displayed high thigmotaxis; track 9 and 10 showed the preference for the north side of the pool (right Qd 1 and left Qd 4). Right panel, Wt having 60 sec latency also showed thigmotaxis. Compare tracks that resulted in hitting goal [hg of Qd3 (south left)], track 15/16 pair at ~40 s (right side top corner number); 17/18 pair at ~20s; and 19/20 pair at ~5s. KO mice often swam parallel to the wall right after release and showed thigmotaxis but Wt would swim away from the wall.
In the probe test (Fig. 4B), both control and MPH-treated WT mice showed preference for quadrant 3 where the hidden platform was located previously. The preference of higher than 25% (the chance level) positively indicated their learning of the task [One-sample t-test, control WT: t(11)=2.465, p<0.05; MPH-treated WT: t(11)=1.998, p<0.05]. One-way ANOVA for control WT also showed F(3,68)=5.290, p=0.002, that percent time in target quadrant 3 vs. other quadrants were all p<0.01. As for MPH-treated WT, F(3,68)=2.709, p<0.052, percent time in target quadrant was not all significantly higher than other quadrants. KO mice, neither control nor MPH-treated showed a preference for quadrant 3, instead, they showed affinities for quadrants 1 and 4 and proved to be poor learners. It is interesting to point out that quadrants 1 and 4 of KO mice’ preferences were on the side near the computer screen and it was also the side where they would be picked up following the swim (see example of such swimming path in Fig. 4D, tracks 9 and 10).
In examining the swimming paths, it became clear that the KO mice showed strong thigmotaxis during training that hindered their abilities to locate the platform. Thigmotaxis in the water maze denotes the tendency of the animal to swim closely to the wall (~15 cm to the wall; about ½ of the total pool surface area). In fact, many KO mutants would swim mainly clinging to the wall in the early blocks of trials (see Fig. 4D, tracks 1 & 2). Some of them would persist in swimming in such a pattern throughout the entire training (track 8, an example of a 36th and last trial). Quantification of thigmotaxis is shown in Fig. 4C as % wall time measuring the fraction of swim time in the outer rim along the wall as shown in Fig. 4D. Tracks 1–7 showed example swim paths of KO mice that lasted 60 sec without hitting goal but exhibiting high thigmotaxis. WT mice rarely showed 100% wall time but they did show some degree of thigmotaxis in the early blocks of trial (60 sec latency, tracks 11–14). WT mice learned fast and hit goal early in the training trials; once it occurred in <30–40 sec, they showed much reduced thigmotaxis (tracks 15, 17 & 19). On the other hand, strong thigmotaxis persisted among the KO mice, even when they did hit the goal (compare tracks of 15/16, 17/18 and 19/20 pairs). One-way repeated measures ANOVA indicated that WT control, F(11,66)=13.313, p<0.0001 and WT+MPH, F(11,55)=13.299, p<0.0001] exhibited significantly decreased thigmotaxis along the blocks but not those of KO mice, either control or MPH-treated. Two-way multiple measures ANOVA did not distinguish MPH treated WT or KO from their respective control cohort, but there were significant differences between WT and KO, both in control and MPH-treated groups, control: F(1,14)=21.463, p<0.0001; MPH-treated: F(1,9)=101.276, p<0.0001.
Effect of MPH treatment on LTP of Ng mutant mice under enrichment condition
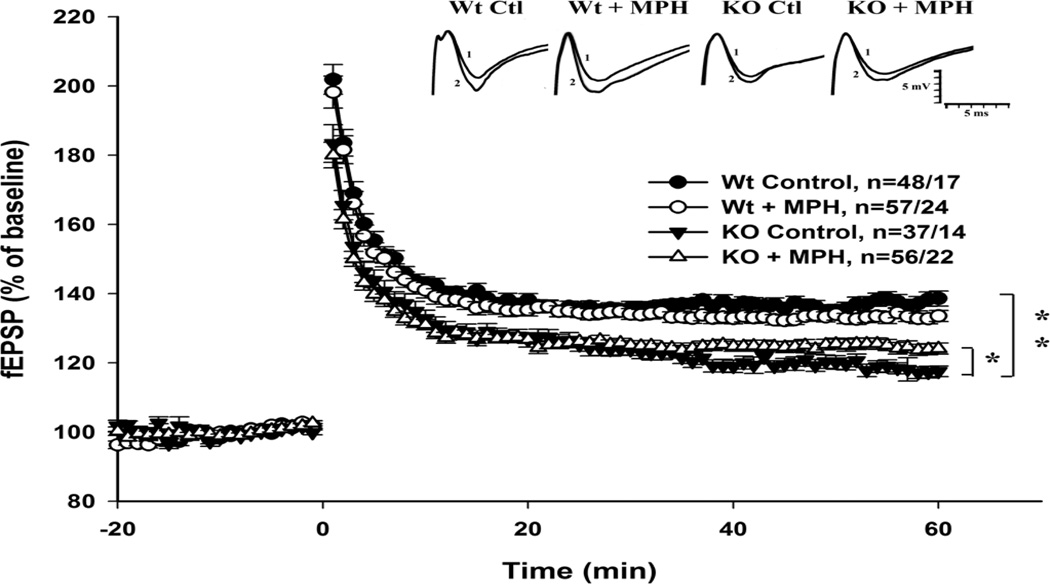
A single 1s, 100 Hz stimulation protocol elicited a smaller LTP in the hippocampal CA1 of NgKO mice comparing to their WT litter mates. Under enrichment conditions, the same induction paradigm enhanced LTP significantly in WT, but did not influence that of mutant mice (Huang et al. 2006). In the present experiment, recordings were on animals that had undergone an extended enrichment period (at least 3 weeks before behavioral testing plus two weeks of testing period). One-way ANOVA on data of averaged last 10-min blocks of the 60 min recordings revealed that the extent of potentiation among groups were significantly different [F(3,36)=942.502, p<0.001]. Post hoc Holm-Sidak pairwise comparison showed that NgKO mice’ potentiation was significantly lower (p<0.01) than that of WT under the same condition (Fig. 5). With the drug treatment, NgKO mice showed a small increase in the extent of potentiation, though small, the increment was significant (p < 0.05). On the other hand, potentiation of MPH-treated WT mice decreased slightly from that of the WT control, however, the decrease was not significant (p = 0.05).
Figure 5.
Effect of MPH treatment on HFS-induced LTP at CA1 region of hippocampus. After HFS of 1s at 100 Hz, KO mice showed lower level of post tetanic potentiation than that of Wt, and the potentiation was further subsided. Comparing the level of potentiation 60 min post HFS, KO was significantly lower than that of Wt. MPH treatment did not alter LTP of Wt, it significantly increased LTP of KO mice over that of untreated KO mice. Representative traces of fEPSP before (1) and 60 min after (2) HFS are shown with calibrations, 5 mV and 5 msec.
Effect of MPH on the hippocampal protein expression
Previous experiments (Huang et al. 2007; Huang et al. 2006) showed that EE enhances several signaling proteins’ expression in hippocampus including both Ng and αCaMKII. The expression pattern as influenced by MPH treatment under enriched conditions is shown in Fig. 6. Among the proteins probed, several displayed distinctive expression differences in their immunoblot profiles either between WT and NgKO or between MPH-treated and untreated samples as shown in Fig. 6A. The relative level of expression, when normalized with actin and compared against WT control samples, is shown in Fig. 6B. In WT samples, MPH treatment caused a significant reduction in CaM, Ng (p<0.01), and PSD95 (p<0.05) expression; levels of PKCε and phosphorylated-αCaMKII also showed tendencies of reduction. In NgKO hippocampus, MPH caused a significant reduction in PKCε (p<0.01) but a great tendency of increase in the GFAP expression (also see immunohistochemistry in the next figure for GFAP). With the exception of PKCε, most of the proteins reduced following MPH treatment were identified as post synaptic proteins considered important regulators for memory formation. Between WT and KO samples, CaM, phosphorylated-αCaMKII (p<0.001), and PSD95 (p<0.01), the signaling molecules of postsynaptic NMDA receptor activation, were significantly reduced in NgKO samples. Reduction in phosphorylated-αCaMKII has been considered as the major culprit of cognitive impairment of the NgKO mice (Pak et al. 2000). There was an increase in GluR1 expression in NgKO, which likely was an adaptation to enhance synaptic transmission in the face of retarded NMDA receptor-mediated signaling. Of particular interest, there was a near 50% increase of GFAP in NgKO comparing to WT (Fig. 6B, also see Fig. 7), though the expression level was quite variable from sample to sample in NgKO hippocampus (see Fig. 6A). It seems likely that there is a great expansion of astrocytes in the NgKO hippocampus as GFAP is a marker protein of them.
Figure 6.
Immunoblot analysis of hippocampal protein expression and quantification. (A) Hippocampal protein samples, 4 to 5 samples of each experimental group, were analyzed on SDS/PAGE. Similar gels were performed 5 times with 24 controls of Wt and KO, and 25 MPH-treated Wt and KO samples. (A) Representative immunoblots showed the relative immunoreactivities of those antibodies visibly exhibiting differences among the groups of animals. Each blot was scanned and the relative intensities were normalized against that of actin (not shown) and compared to the Wt control sample in the same blot. Taking Wt control as 100%, relative values of all groups were plotted in (B): n=24 for Wt and KO control; n=25 for MPH-treated Wt and KO; ***p<0.001; **p<0.01; *p<0.05.
GFAP and CaM expression in hippocampus as affected by MPH treatment
The increase in GFAP expression observed in NgKO hippocampus was best exemplified in the immunohistochemical staining of hippocampal sections (Fig. 7A). The figure shows two regions of the hippocampal formation, the dentate gyrus (A to D) and CA1 (E to H), all were from the similar locations of hippocampus, as captured by a 20× objective lens in a parallel analysis. In both regions, positively stained astrocytes in KO were significantly greater than seen in WT (compare C over A and G over E). It was also apparent that MPH treatment had further increased the GFAP-positive astrocytes in NgKO mice in these two hippocampal regions (compare D over C and H over G). This was in contrast to the immunoblot analysis (Fig. 6) where whole hippocampal protein was compared. Although NgKO did show higher levels of GFAP than WT, it was not significantly affected by MPH treatment. This might be explained by the fact that GFAP-immunohistochemical analysis, unlike immunoblot, detects only a small population of reactive astrocytes and neuronal progenitor radial glial cells but not all mature glial cells (Sofroniew & Vinters 2010). Relative number of GFAP-positive profiles at the whole area of hilus or central CA1 stratum radiatum as measured in all groups were summarized in the bar graph (Fig. 7B). One-way ANOVA for GFAP at dentate gyrus, F(3,29)=48.444, p<0.001; at CA1, (F3,29)=11.993, p<0.001. As for the CaM expression, it was localized majorly in the pyramidal cell layer of CA1 (I to L in Fig. 7A) and granule cell layer of dentate gyrus (not shown). Both immunoblot and immunohistochemical staining all indicated that WT expressed much higher level of CaM than KO. However in the immunostaining, analyzing the cell layer only, MPH showed positive effects on CaM expression in both groups of animal, whereas, in immunoblot analysis of the whole hippocampus, MPH exhibited negative effect. It is possible that MPH treatment has further retained CaM at the cell layer (Huang et al. 2011). One-way ANOVA for CaM staining at CA1, F(3,62)=319.372, p<0.001. Changes of CaM expression in granule cell layer of dentate gyrus were similar to that of pyramidal cell layer at CA1 (data not shown).
Discussion
Results of the present series of experiments show that NgKO mice performed poorly in contextual fear conditioning and Morris water maze even after an extended period of exposure to an enriched environment. The results also show that these mutant mice were hyperactive and they lacked social interaction ability. Taken together, this abnormal behavior pattern and cognitive defects of Ng KO mice resembled those children diagnosed as Jacobsen syndrome with chromosome 11q terminal deletion including BSX and Ng genes (Coldren et al. 2009). We also observed improvement of behavior and cognitive function in these mutant mice by MPH treatment under enrichment condition.
When first placed in a novel environment, most rodents tend to be curious and explore more which manifests as an increase in locomotor activity. Compared to WT, the hyperactivity of the NgKO mice appears to surpass mere curiosity. Such hyper-locomotor activity of rodent and traveling at marginal edges of the activity box, also observed in NgKO mice, were often thought to associate with the anxiety-related behavior (Prut & Belzung 2003). However, the present forced-swim and elevated plus maze tests disclosed none of the depressive or anxiety-related behavior but hyperactivity in these mice. So far we have not used a more specific test to evaluate directly their inattentive nature or impulsive behavior, the other characteristic of ADHD children. Previously, when these mutant mice were tested on the radial-arm maze (Huang et al. 2007; Huang et al. 2006), even after 20 hrs of food deprivation, the mutant mice would enter each arm sequentially without any intention of consuming the bait, though they did during the acclimation period when bait was plentiful and throughout the arm. During the present elevated plus maze test, the mutant mice would travel in and out of the arm, and repeatedly looking over the edge of the open arm. Some did jump off the maze despite the height of the maze. In the Morris water maze, these mutant mice swam largely in a thigmotactic manner ignoring the escaping platform, and struggled not to be placed on it or jumped and swam away when they accidently landed on it. All these genotype-specific peculiarities of behavior were rarely observed in the WT mice. After extended enrichment and MPH treatment, the hyperactive and abnormal behaviors of the mutant seemed less apparent, and they made improvement in the tests.
MPH is often prescribed for treating ADHD children. As was noted, it might also improve performance and decrease motor restlessness for many in the general population (Aman et al. 1984; Hellwig-Brida et al. 2011). As the behavior of NgKO mice is very much ADHD-like and, previously, environmental enrichment alone failed to improve their behavior or cognitive function, MPH treatment for these mice was thought to be the obvious choice. But in our initial trial MPH alone did not affect the behavior of these mutant mice, besides, MPH appeared to have adverse effect on WT mice. For example, MPH treatment caused WT mice to become more active and perform poorer as compared to control mice. Hyperactivity induced in WT mice by psycho-stimulant drugs has been reported (Yan et al. 2009). Housing the mice in an enriched environment during MPH treatment produced a positive effect that was beyond simple calming. The effect of MPH on mutant mice varied greatly both on motor activity and cognitive behavior. Children of Jacobsen syndrome, having chromosome11q terminal deletion including Ng gene, show lower IQ scores (Coldren et al. 2009) and abnormal behavior, but it is not known whether they were also low in dopamine transmission. We have not measured dopamine level in the NgKO mutant either. As MPH is a dopamine reuptake inhibitor, it would be interesting to monitor dopamine as well as other monoamine transmitters in regions of interest like striatum, prefrontal cortex and hippocampus in the NgKO mice. It is likely that the NgKO mice and 11q-children, though showing ADHD-like behavior but not ADHD genetically, would not get better by mere MPH treatment.
The hippocampal protein expression patterns showed mainly reductions of post synaptic proteins, such as CaM, αCaMKII, and PSD95, as expected from down-regulated post NMDA receptor signalings resulting from Ng deletion. However, these molecular defects were not corrected in MPH-treated hippocampus; thus, mechanistic explanation for the behavioral or cognitive improvement of the treated NgKO mice was not fully uncovered. MPH did positively increase the presence of CaM at pyramidal cell layer of CA1 and granular cell layer of dentate gyrus. How the elevated CaM, in the absence of Ng, promotes cognitive function is presently unknown. MPH was recently reported to directly amplify LTP in hippocampus via noradrenergic mechanisms (Dommett et al. 2008). In our experiment LTP was significantly improved in hippocampus of MPH treated mutant mice that might have contributed to the behavioral improvement. It seemed that MPH might act through the elevation of dopamine (or noradrenalin) and, under the enrichment condition, activated signaling pathways that improved behavioral deficits. Though MPH treatment under environmental enrichment did improve the behavior and cognitive deficit of these mutant mice, it did not reverse completely their defects compared to WT mice. Probably, the lack of the Ng-modulated pathway in NgKO mice is still the root cause for the non-complete reversal. It would be interesting to test the combination of MPH with agents that bypass Ng in enhancing NMDA receptor’s downstream signaling reactions for the facilitation of memory formation. A possible strategy is to use activators of PKC or PKA, which have been tested positively in enhancing LTP in our electrophysiological studies using these mutant mice (F. L. Huang and K.-P. Huang, unpublished data).
Up-regulation of GFAP through repeated treatment of MPH has been reported (Cavaliere et al. 2012) and was viewed as an expansion of reactive astrocytes, which eventually affect glutamate receptor sensitization and increase of dendritic spine number. These authors suggested an adaptation model via astrocytic morpho-functional rearrangement. Recently it was recognized that some glial cells during development and certain specific subpopulations of astrocytes in adult were in fact primary progenitors or neural stem cells (Kriegstein & Alvarez-Buylla 2009). It is possible that an expansion of astrocytes in subgranular zone, and more specifically, the newly formed radial glial cells may have potential for neurogenesis (Gage 2000; Seri et al. 2004). It requires many more analysis regarding if any of the presently observed increase of GFAP-positive cells in the MPH treated NgKO mice also express characteristics of proliferating neuron markers (Kempermann 2002). It is interesting, however, that the increase of GFAP-positive cells occurs not only within the dentate gyrus but also at the CA1 region, where neurogenesis is not normally considered occurring. In this regard, the aforementioned MPH/astrocyte activation/synaptic remodeling scheme (Cavaliere et al. 2012) is much more favorable. It was also a surprise to observe that control NgKO mice already displayed 40–50% more GFAP in hippocampus than their WT counterparts. It was possible that these mutant mice, lacking Ng and having down-regulated NMDA receptor-mediated signaling for memory acquisition, exerted a long-term adaptation strategy for the compensation of their deficits. It’s worthwhile to mention that, though, short term EE showed no beneficial effect on these mice (Huang et al. 2006), positive effect of long-term EE (Huang et al. 2007) on the aging mutant mice could have been the accumulation of such long-term hippocampal modifications.
In summary, this is the first time we were able to improve the cognitive behaviors of these mutant mice. In light of certain similarities in behavioral phenotypes between NgKO mice and those of Jacobsen syndrome children with haploid-insufficiency in Ng, MPH under an enriched environment could be beneficial to those patients. NgKO mice could be used for the future study and development of new drug treatments. Mechanistically, the revelation that NgKO mice display higher levels of GFAP than WT and that MPH treatment causes a further elevation prompt many future studies regarding the expansion of astrocytes in other NMDA receptor signaling compromised brains.
Acknowledgement
This work was supported by the Intramural Research Program of National Institute of Child Health and Human Development, National Institutes of Health. The authors like to thank Daniel Abebe, James Ofori, Bahafta Behre and Dennis Costellon for their helps in the behavioral test and animal welfare including maintenance of enrichment environment and drug injection. We also like to thank Dr. Chris McBain, Program Chief of Developmental Neurobiology, NICHD, for his critical reading and suggestion regarding the manuscript.
Footnotes
The authors have no conflict of interest to declare
References
- Aman MG, Vamos M, Werry JS. Effects of methylphenidate in normal adults with reference to drug action in hyperactivity. Aust N Z J Psychiatry. 1984;18:86–88. doi: 10.3109/00048678409161040. [DOI] [PubMed] [Google Scholar]
- Cavaliere C, Cirillo G, Bianco MR, Adriani W, De Simone A, Leo D, Perrone-Capano C, Papa M. Methylphenidate administration determines enduring changes in neuroglial network in rats. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22:53–63. doi: 10.1016/j.euroneuro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Coldren CD, Lai Z, Shragg P, Rossi E, Glidewell SC, Zuffardi O, Mattina T, Ivy DD, Curfs LM, Mattson SN, Riley EP, Treier M, Grossfeld PD. Chromosomal microarray mapping suggests a role for BSX and Neurogranin in neurocognitive and behavioral defects in the 11q terminal deletion disorder (Jacobsen syndrome) Neurogenetics. 2009;10:89–95. doi: 10.1007/s10048-008-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett EJ, Henderson EL, Westwell MS, Greenfield SA. Methylphenidate amplifies long-term plasticity in the hippocampus via noradrenergic mechanisms. Learn Mem. 2008;15:580–586. doi: 10.1101/lm.1092608. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gerendasy DD, Sutcliffe JG. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Molecular neurobiology. 1997;15:131–163. doi: 10.1007/BF02740632. [DOI] [PubMed] [Google Scholar]
- Grossfeld PD, Mattina T, Lai Z, Favier R, Jones KL, Cotter F, Jones C. The 11q terminal deletion disorder: a prospective study of 110 cases. Am J Med Genet A. 2004;129A:51–61. doi: 10.1002/ajmg.a.30090. [DOI] [PubMed] [Google Scholar]
- Hellwig-Brida S, Daseking M, Keller F, Petermann F, Goldbeck L. Effects of methylphenidate on intelligence and attention components in boys with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:245–253. doi: 10.1089/cap.2010.0041. [DOI] [PubMed] [Google Scholar]
- Huang FL, Huang KP, Boucheron C. Long-term enrichment enhances the cognitive behavior of the aging neurogranin null mice without affecting their hippocampal LTP. Learn Mem. 2007;14:512–519. doi: 10.1101/lm.636107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Jager T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004;24:10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Li J, Schuck P, McPhie P. Calcium-sensitive interaction between calmodulin and modified forms of rat brain neurogranin/RC3. Biochemistry. 2000;39:7291–7299. doi: 10.1021/bi000336l. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Shetty PK. Stimulation-mediated translocation of calmodulin and neurogranin from soma to dendrites of mouse hippocampal CA1 pyramidal neurons. Neuroscience. 2011;178:1–12. doi: 10.1016/j.neuroscience.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir O, Tabbane K, Sengupta S, Joober R. Candidate genes and neuropsychological phenotypes in children with ADHD: review of association studies. J Psychiatry Neurosci. 2009;34:88–101. [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pak JH, Huang FL, Huang KP. N-methyl-D-aspartate induces neurogranin/RC3 oxidation in rat brain slices. J Biol Chem. 1999;274:1294–1300. doi: 10.1074/jbc.274.3.1294. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Edds JS, Young WS., 3rd Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nat Protoc. 2009;4:1574–1581. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, Westphal H, Huang KP. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II. synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci U S A. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RD, Takahashi RN. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: a rodent model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2009;20:134–145. doi: 10.1097/FBP.0b013e32832a80bf. [DOI] [PubMed] [Google Scholar]
- Prichard L, Deloulme JC, Storm DR. Interactions between neurogranin and calmodulin in vivo. J Biol Chem. 1999;274:7689–7694. doi: 10.1074/jbc.274.12.7689. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis PKA. MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. The Journal of comparative neurology. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Sheu FS, Mahoney CW, Seki K, Huang KP. Nitric oxide modification of rat brain neurogranin affects its phosphorylation by protein kinase C and affinity for calmodulin. J Biol Chem. 1996;271:22407–22413. doi: 10.1074/jbc.271.37.22407. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Zhao J, Miller LR, Cheng SY, McDonald MP. Transgenic mice expressing a human mutant beta1 thyroid receptor are hyperactive, impulsive, and inattentive. Genes Brain Behav. 2006;5:282–297. doi: 10.1111/j.1601-183X.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TC, Hunt SP, Stanford SC. Behavioural and neurochemical abnormalities in mice lacking functional tachykinin-1 (NK1) receptors: a model of attention deficit hyperactivity disorder. Neuropharmacology. 2009;57:627–635. doi: 10.1016/j.neuropharm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Yan TC, McQuillin A, Thapar A, Asherson P, Hunt SP, Stanford SC, Gurling H. NK1 (TACR1) receptor gene 'knockout' mouse phenotype predicts genetic association with ADHD. Journal of psychopharmacology. 2010;24:27–38. doi: 10.1177/0269881108100255. [DOI] [PMC free article] [PubMed] [Google Scholar]