Abstract
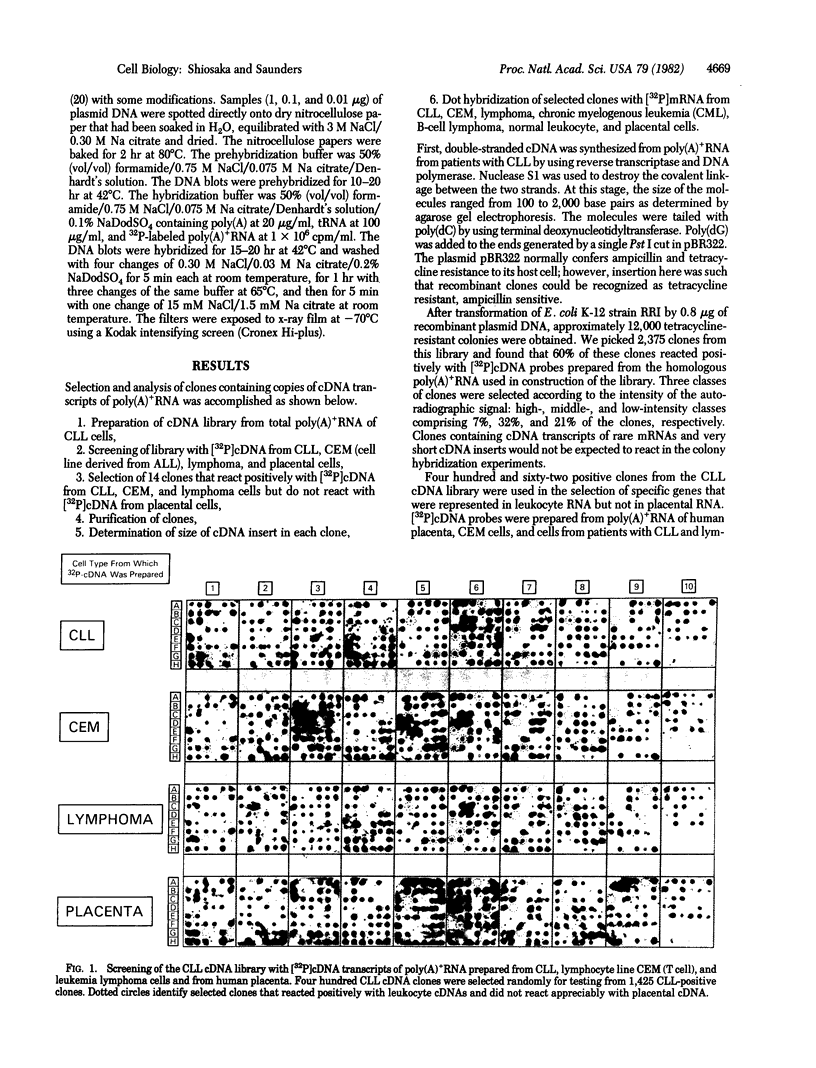
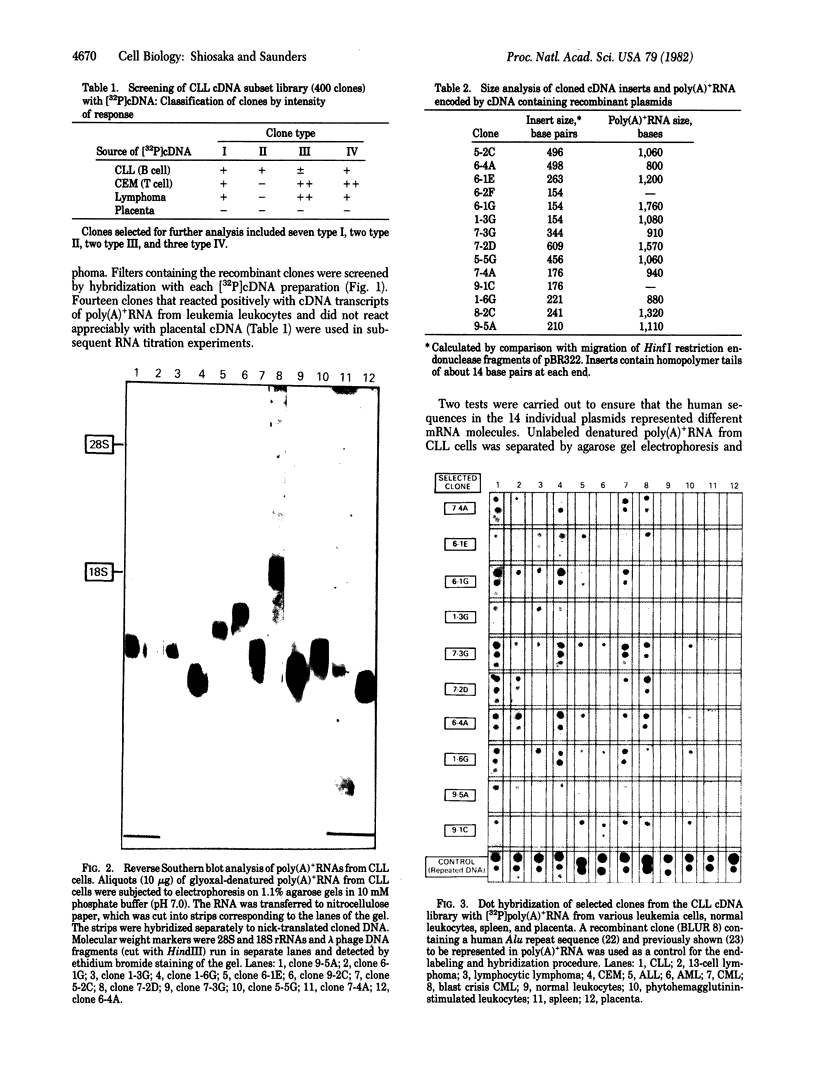
cDNA clones complementary to mRNA of cells from patients having chronic lymphocytic leukemia (CLL) were used to examine quantitative changes in the mRNA levels of specific genes in human leukemia leukocytes. Fourteen (of 400) CLL-positive clones that did not hybridize with placental mRNA were studied. Three of the 14 clones were highly represented in mRNA from CEM, a T-cell line. One clone was highly represented in mRNA from CLL and two were highly represented in mRNA from patients with chronic myelocytic leukemia. Ten clones were not significantly represented in normal leukocytes and spleen mRNA. We have identified several genes that are differentially expressed in human leukemia leukocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atryzek V., Tamaoki T., Fausto N. Changes in polysomal polyadenylated RNA and alpha-fetoprotein messenger RNA during hepatocarcinogenesis. Cancer Res. 1980 Oct;40(10):3713–3718. [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Maizel A. L., Saunders G. F. mRNA in human cells contains sequences complementary to the Alu family of repeated DNA. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6003–6007. doi: 10.1073/pnas.78.10.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M., Deeb S. S., Sueoka N. Sequence complexity of nuclear RNAs in adult rat tissues. Cell. 1978 Jan;13(1):111–120. doi: 10.1016/0092-8674(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier M. L., Montagna R. A., Saunders G. F. Insulin gene expression during development of the fetal bovine pancreas. Biochemistry. 1981 Jan 20;20(2):367–371. doi: 10.1021/bi00505a022. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Spiegelman S., Franklin N. C., Luria S. E. SEPARATION OF THE RNA MESSAGE TRANSCRIBED IN RESPONSE TO A SPECIFIC INDUCER. Proc Natl Acad Sci U S A. 1963 May;49(5):729–736. doi: 10.1073/pnas.49.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Humphreys T. Similarity of hnRNA sequences in blastula and pluteus stage sea urchin embryos. Cell. 1977 Sep;12(1):143–155. doi: 10.1016/0092-8674(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Ried M. E., Saunders G. F. Abundancy and diversity of mRNA sequences in human leukocytes. Differentiation. 1976 Jun 4;5(2-3):175–178. doi: 10.1111/j.1432-0436.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Cell-free modulation of proinsulin synthesis. Science. 1977 Nov 11;198(4317):620–622. doi: 10.1126/science.918657. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Dahlberg J. E., Szybalski W. Processing of the major leftward mRNA of coliphage lambda. Virology. 1976 May;71(1):262–277. doi: 10.1016/0042-6822(76)90111-2. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Grady D. L., Li D. W., Mirvis S. E., Ts'o P. O. Extensive homology of nuclear ribonucleic acid and polysomal poly(adenylic acid) messenger ribonucleic acid between normal and neoplastically transformed cells. Biochemistry. 1980 Mar 4;19(5):821–832. doi: 10.1021/bi00546a001. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Supowit S. C., Rosen J. M. Gene expression in normal and neoplastic mammary tissue. Biochemistry. 1980 Jul 22;19(15):3452–3460. doi: 10.1021/bi00556a008. [DOI] [PubMed] [Google Scholar]
- Tashima M., Calabretta B., Torelli G., Scofield M., Maizel A., Saunders G. F. Presence of a highly repetitive and widely dispersed DNA sequence in the human genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1508–1512. doi: 10.1073/pnas.78.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B. J., Klein W. H., Hough-Evans B. R., Britten R. J., Davidson E. H. Sea urchin embryo mRNA sequences expressed in the nuclear RNA of adult tissues. Cell. 1978 Aug;14(4):941–950. doi: 10.1016/0092-8674(78)90348-3. [DOI] [PubMed] [Google Scholar]