Abstract
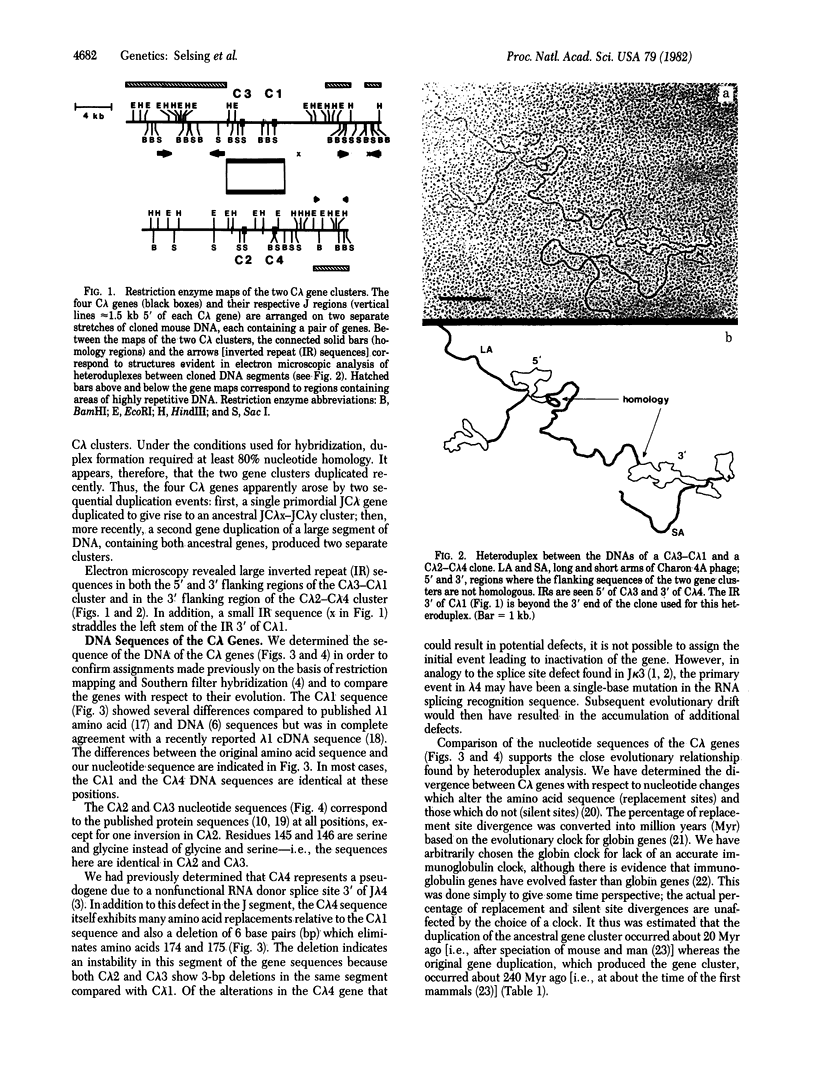
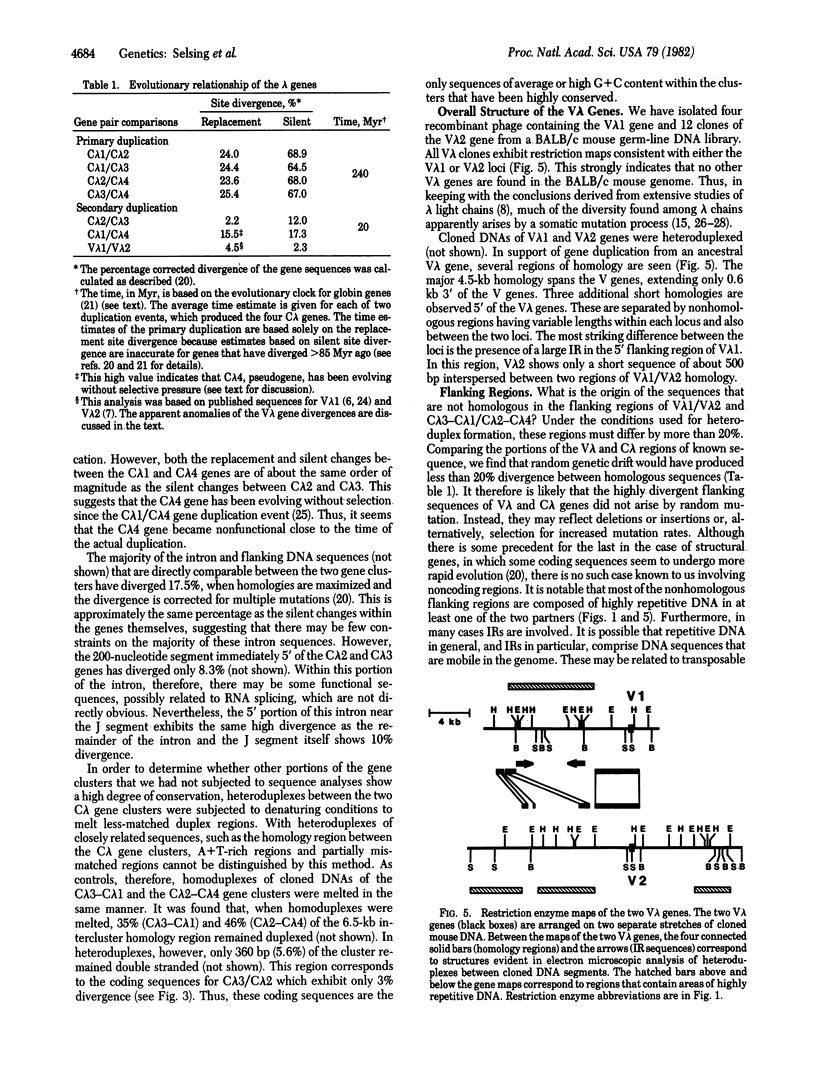
The mouse has four C lambda and two V lambda genes. We have isolated Charon 4A clones that contain all six lambda genes from a BALB/c germ-line library. We present here the DNA sequences of the C lambda 2, C lambda 3, and C lambda 4 genes and also correct what are apparently errors in previous reports of C lambda 1 protein and DNA sequences. In addition, we have analyzed cloned DNAs by restriction mapping and electron microscopy to determine the relationships among the various lambda genes. By heteroduplex analysis, two gene clusters containing JC lambda 3--JC lambda 1 and JC lambda 2--JC lambda 4 show homology extending from the J regions 5' of C lambda 3/C lambda 2 to just 3' of C lambda 1/C lambda 4. Other than the region between the genes, very little homology exists in the C lambda flanking regions. In contrast, V lambda 1 and V lambda 2 genes show considerable homology extending into the 5' flanking regions. Large inverted repeats are found in the 5' flanking regions of V lambda 1 and C lambda 3, as well as in the 3' flanking regions of both C lambda gene clusters. DNA sequence divergences between the C lambda genes indicate that an ancestral JC lambda x--JC lambda g gene cluster arose at about the time of the first mammals by duplication of a primordial JC lambda gene. The data further suggest that the JC lambda x--JC lambda gene cluster duplicated after the speciation of mouse and man and subsequently diverged into the present day JC lambda 3--JC lambda 1 and JC lambda 2--JC lambda 4 gene clusters. C lambda 4, a pseudogene, became inactive at about the time of duplication of the ancestral JC lambda x--JC lambda y cluster. Comparison of DNA sequence divergence between the V lambda 1 and V lambda 2 genes demonstrates an anomaly. The percentage of amino acid replacement changes is approximately the same for V lambda 1/V lambda 2 as for C lambda 3/C lambda 2, implying that the ancestral V lambda gene was duplicated at the same time, and possibly together with, the JC lambda x--JC lambda y cluster. However, there are fewer silent changes than amino acid replacement changes between the V lambda 1/V lambda 2 genes, suggesting either that a selective pressure acted on the silent sites or that V lambda genes have only recently been duplicated. We also consider the possibility of a gene conversion event subsequent ot a more ancient duplication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Steiner L. A., Eisen H. N. Identification of a third type of lambda light chain in mouse immunoglobulins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):569–573. doi: 10.1073/pnas.78.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Traunecker A., Eisen H., Tonegawa S. Organization of four mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3765–3769. doi: 10.1073/pnas.78.6.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Grant J. A., Sanders B., Hood L. Partial amino acid sequences of chicken and turkey immunoglobulin light chains. Homology with mammalian lambda chains. Biochemistry. 1971 Aug 3;10(16):3123–3132. doi: 10.1021/bi00792a022. [DOI] [PubMed] [Google Scholar]
- Lacy E., Maniatis T. The nucleotide sequence of a rabbit beta-globin pseudogene. Cell. 1980 Sep;21(2):545–553. doi: 10.1016/0092-8674(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Bothwell A., Storb U. Physical linkage of the constant region genes for immunoglobulins lambda I and lambda III. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3829–3833. doi: 10.1073/pnas.78.6.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Selsing E., Storb U. Structural alterations in J regions of mouse immunoglobulin lambda genes are associated with differential gene expression. Nature. 1982 Feb 4;295(5848):428–430. doi: 10.1038/295428a0. [DOI] [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Potter M., Rudikoff S., Vrana M., Rao D. N., Mushinski E. B. Primary structural differences in myeloma proteins that bind the same haptens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):661–666. doi: 10.1101/sqb.1977.041.01.075. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Storb U., Selsing E., Zentgraf H. Comparison of different rearranged immunoglobulin kappa genes of a myeloma by electronmicroscopy and restriction mapping of cloned DNA: implications for "allelic exclusion". Nucleic Acids Res. 1980 Oct 24;8(20):4689–4707. doi: 10.1093/nar/8.20.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Riblet R. Genetic control of antibody variable regions. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):837–846. doi: 10.1101/sqb.1977.041.01.093. [DOI] [PubMed] [Google Scholar]



