Abstract
Antiviral activity of rabbit and mouse fibroblast interferons was irreversibly destroyed by treatment with halomethyl ketone derivatives of phenylalanine but not by treatment with a halomethyl ketone derivative of lysine. The inactivation reaction was pH dependent, suggesting the involvement of an amino acid residue ionizing in the region of pH 7. Tryptophan and phenylalanine, known ligands of interferons, protected rabbit interferon substantially against inactivation by the chloromethyl ketone derivative of N-tosylphenylalanine. Mixed bovine brain gangliosides protected rabbit and mouse interferons against inactivation by this reagent. Although halomethyl ketone derivatives of phenylalanine were originally designed and used for affinity labeling of the active site of chymotrypsin and similar enzymes, no evidence was found for a chymotrypsin-like activity of interferons. It is proposed that halomethyl ketone derivatives of phenylalanine inactivate interferon by an affinity labeling mechanism, first binding to a hydrophobic binding site and then reacting irreversibly with a nearby nucleophilic amino acid residue, which appears to be a histidine. This conclusion implies that a hydrophobic site on interferons is necessary for their antiviral activity.
Full text
PDF
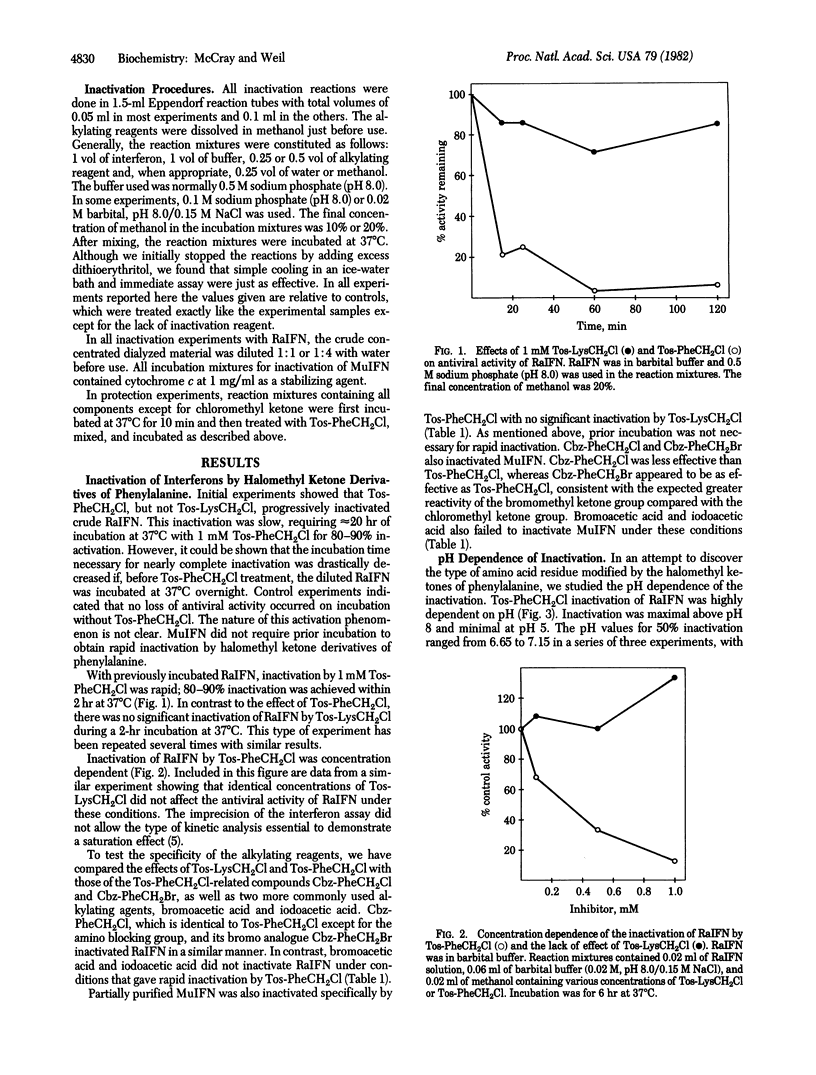
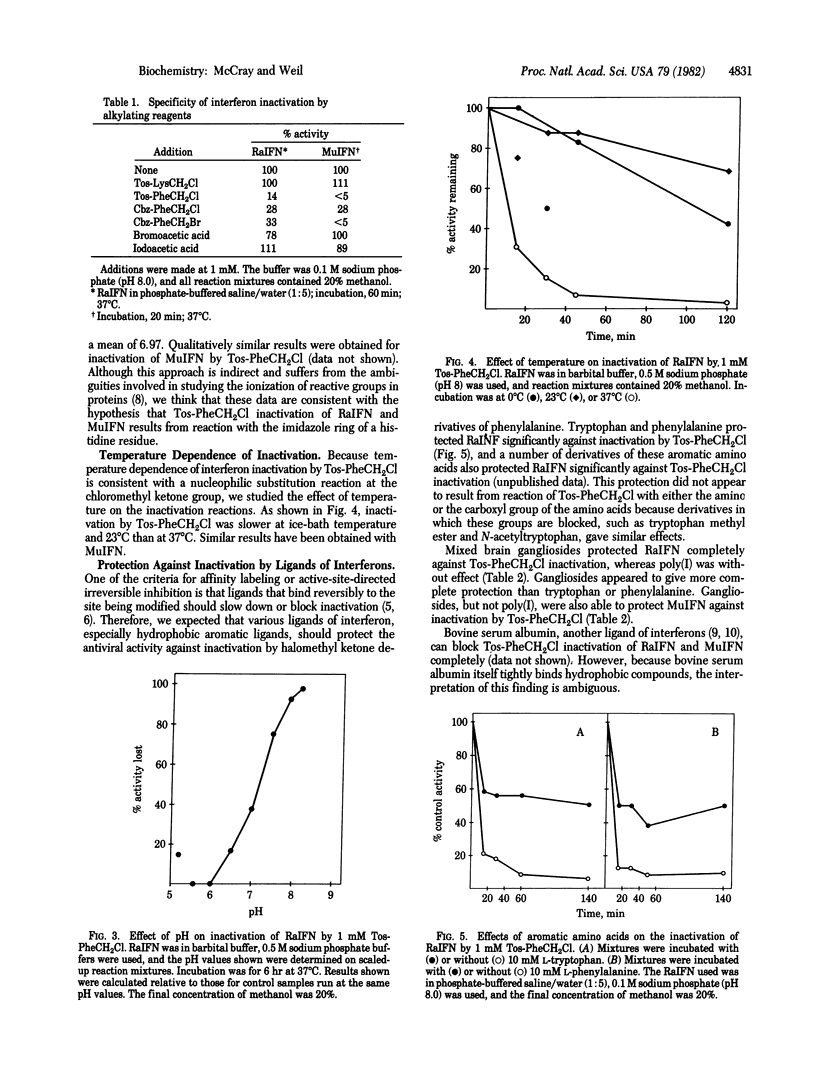


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besançon F., Ankel H. Membrane receptors for interferon. Tex Rep Biol Med. 1977;35:282–292. [PubMed] [Google Scholar]
- Brubacher L. J., Bender M. L. The preparation and properties of trans-cinnamoyl-papain. J Am Chem Soc. 1966 Dec 20;88(24):5871–5880. doi: 10.1021/ja00976a032. [DOI] [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Purification and characterization of mouse interferon with novel affinity sorbents. J Virol. 1976 Feb;17(2):439–445. doi: 10.1128/jvi.17.2.439-445.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer-Guignard J., Thang M. N., De Maeyer E. Binding of mouse interferon to polynucleotides. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3787–3790. doi: 10.1073/pnas.74.9.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner F., Scriba M., Weil R. Interferon: evidence for its glycoprotein nature. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1981–1985. doi: 10.1073/pnas.70.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. W., Davey M. W., Hejna C. J., Von Muenchhausen W., Sulkowski E., Carter W. A. Selective binding of human interferon to albumin immoblized on agarose. J Biol Chem. 1974 Jul 25;249(14):4665–4667. [PubMed] [Google Scholar]
- Kupfer A., Gani V., Jiménez J. S., Shaltiel S. Affinity labeling of the catalytic subunit of cyclic AMP-dependent protein kinase by N alpha-tosyl-L-lysine chloromethyl ketone. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3073–3077. doi: 10.1073/pnas.76.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., McElroy W. D. Role and reactivity of sulfhydryl groups in firefly luciferase. Biochemistry. 1969 Jan;8(1):130–136. doi: 10.1021/bi00829a018. [DOI] [PubMed] [Google Scholar]
- Niinobe M., Hitomi Y., Fujii S. A sensitive colorimetric assay for various proteases using naphthyl ester derivatives as substrates. J Biochem. 1980 Mar;87(3):779–783. doi: 10.1093/oxfordjournals.jbchem.a132807. [DOI] [PubMed] [Google Scholar]
- Powers J. C. Reaction of serine proteases with halomethyl ketones. Methods Enzymol. 1977;46:197–208. doi: 10.1016/s0076-6879(77)46020-8. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Sulkowski E., Davey M. W., Carter W. A. Interaction of human interferons with immobilized hydrophobic amino acids and dipeptides. J Biol Chem. 1976 Sep 10;251(17):5381–5385. [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]
- Wold F. Affinity labeling--an overview. Methods Enzymol. 1977;46:3–14. doi: 10.1016/s0076-6879(77)46005-1. [DOI] [PubMed] [Google Scholar]


