Abstract
Aims: Tissue inhibitors of metalloproteinase (TIMPs) bind to active matrix metalloproteinase (MMPs), and thereby inhibit their proteolytic activity. We investigated the role of polymorphisms in the gene for TIMP-1 and serum levels of TIMP-1 in association with postmyocardial infarction (MI), left ventricular (LV) dysfunction, and symptoms of acute heart failure (AHF) in patients treated with primary percutaneous coronary intervention. Methods: In total, 556 patients with STEMI were evaluated. Levels of TIMP-1 were measured at admission and 24 h after MI onset. The TIMP-1 exon 5 SNP rs4898 (F124F with T>C) located at X chromosome was assayed. Results: TIMP-1 levels were higher for men with AHF as well as for men with LV dysfunction (ejection fraction [EF]<40%). According to multivariate analysis, the TIMP-1 level was a factor with an independent negative relationship to EF and AHF in men. An independent relationship between exon 5 TIMP-1 gene polymorphism and EF, AHF or TIMP-1 level was not documented. Conclusion: These results provide evidence that a higher level of circulating TIMP-1 is independently associated with worse EF and AHF.
Introduction
The extracellular matrix (ECM) of the myocardium is a dynamic network of proteins and proteoglycans. The ECM plays a crucial part in the maintenance of ventricular shape, size, and function. The structural integrity relies on a balance between synthesis and degradation of ECM proteins. The physical link between them is mediated by integrins, α/β heterodimeric transmembrane glycoproteins that connect ECM proteins to actin cytoskeleton and play a critical role in transducing bidirectional signals between extracellular and intracellular compartments (Giancotti and Ruoslahti, 1999). Specific changes in the ECM start during myocardial infarction (MI) and after healing determine creation of the postinfarction scar and remodeling. The largest part of the ECM is formed by collagen, which is very stable and its degradation is driven by specific collagenase; however, only 1%–2% of this myocardial collagenase is present in its active form (Tyagi, 1993). After MI, collagenase activity is increased not only in the infarction area, but simultaneously in the rest of the myocardium. The destruction rate of the ECM in infarcted and noninfarcted areas is a leading factor of the size and shape of left ventricle after MI and myocardial systolic performance (Baicu et al., 2003).
Range of the ECM destruction is governed by the balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) activity. The TIMPs family consists of four members: types 1, 2, 3, and 4 (Nagase and Woessner, 1999). TIMP-1 is a 184-amino acid glycoprotein of molecular weight 28.5 kDa located at the short arm of the X chromosome (Xp11.3–p11.23) (Docherty et al., 1985). It was demonstrated that deficiency of TIMP-1 led to significantly increased left ventricular end-diastolic volume (LVEDV) and decreased ejection fraction (EF) after MI in TIMP-1 knockout mice. The accelerated myocardial remodeling induced by deletion of the TIMP-1 gene could be pharmacologically inhibited by matrix metalloproteinase (MMP) inhibitors (Ikonomidis et al., 2005). Increased levels of TIMP-1 in serum were found in patients with MI (Furenes et al., 2009), and the higher levels correlated with left ventricular (LV) dysfunction (Kelly et al., 2008).
The TIMP-1 372T>C polymorphism in exon 5 has been suggested to account for the great majority of polymorphisms in the gene, whereas the other polymorphisms have a very low frequency of occurrence (Hinterseher et al., 2007). Information on the possible influence of polymorphism in the gene for TIMP-1, on serum levels of TIMP-1, and on LV function in the acute phase of MI has not yet been published.
We investigated the role of polymorphisms in the gene for TIMP-1 and serum levels of TIMP-1 in association with post-MI LV dysfunction and acute heart failure (AHF) early after presentation in patients with STEMI treated with primary percutaneous coronary intervention (PCI).
Patients and Methods
Approval of the study protocol
Written informed consent was obtained from all subjects before participation in the study. The study protocol complied with the Declaration of Helsinki. The study protocol was approved by the local Ethics Committee of the University Hospital Brno and by the Ethics Committee of the Masaryk University (Brno, Czech Republic).
Study population
From November 2005 to October 2008, 913 patients with ST segment elevation myocardial infarction (STEMI) were referred for primary PCI. They were admitted to the Coronary Care Unit of the Cardiology Department of the University Hospital Brno.
The STEMI diagnosis was based on symptoms consistent with MI in conjunction with appropriate changes on electrocardiography, that is, ST-segment elevation or new left bundle branch block (LBBB) and elevation in the levels of myocardial necrosis markers (troponin I). Time from the onset of chest pain until primary PCI was <12 h. Standard therapy, that is, angiotensin-converting enzyme inhibitors, beta-blockers, and statins, was given as soon as possible after primary PCI. PCI was undertaken mostly by femoral access with stent implantation. Exclusion criteria were: age >80 years (n=112); known or newly diagnosed malignancy; inflammatory disease or connective-tissue disease; known previous LV dysfunction; estimated life expectancy <12 months (n=48); refusal to provide written consent or noncompliance (n=74); geographic factors (distance from the place of residence to the hospital of >100 km without the possibility of follow-up; n=35). In total, 51 patients were not included because of technical and organizational problems. The TIMP genotypes were not obtained for 37 patients. In total, 556 consecutive Caucasian patients with STEMI were included in the present study. The diagnosis of AHF was assessed according to clinical signs upon hospital admission and/or during hospitalization (Killip class I–IV). Mild AHF was defined as pulmonary congestion with wet rales in the lower half of the lung fields or S3 gallop.
Laboratory methods
Samples of venous blood for analyses of the activity of B-type natriuretic peptide (BNP), N-terminal pro B-type natriuretic peptide (NT-ProBNP), and TIMP-1 were drawn immediately upon hospital admission before primary PCI (sample 1). Blood samples were drawn again 24 h after the onset of chest pain (sample 2). Samples were centrifuged within 20 min in a refrigerated centrifuge, and the plasma and serum were stored at–80°C. Standard biochemical and hematological blood tests were performed immediately upon hospital admission before primary PCI and 24 h after the onset of chest pain (including troponin I; Abbott Laboratories, Abbott Park, IL). BNP was analyzed using the AxSYM BNP-Microparticle Enzyme Immunoassay (Abbott Laboratories). NT-ProBNP was analyzed using the Cobas E411 NT-proBNP Imunoassay Kit (Roche Diagnostics, Indianapolis, IN). The serum level of TIMP-1 was measured in duplicate using a two-site sandwich enzyme-linked immunosorbent assay (ELISA) (Amersham, Buckinghamshire, UK). This assay was used to measure the level of free TIMP-1 as well as TIMP-1 complexed with various MMPs (MMP-1/TIMP-1 complexes). The intra-assay coefficient of variation for TIMP-1 was <5%.
Echocardiographic assessment
Echocardiography was carried out during the index admission (between the third and fifth day after MI onset). Left ventricular end-systolic volume (LVESV), LVEDV, and LV EF were estimated using the bi-planar Simpson's rule from apical two- and four-chamber views, or by the Teichholz formula. Echocardiography was assessed by two operators using the Vivid 7 or Vivid i systems (GE Vingmed Ultrasound, Horten, Norway).
Invasive measurement
With the patient in the supine position, central aortic systolic blood pressure and diastolic blood pressure were measured before LV angiography using a fluid-filled 5-F pigtail catheter before primary PCI. A diseased coronary vessel was defined as one having ≥50% reduction of intraluminal diameter in any of the coronary arteries (left main, left anterior descending, left circumflex, right coronary) or the main branches with a diameter ≥2.0 mm. Significant left main artery stenosis was classified as two-vessel disease.
Genetic analysis
DNA was extracted from peripheral leukocytes using the standard proteinase K technique. The TIMP-1 gene polymorphism on the X chromosome included in the present study was exon 5 SNP rs4898 (F124F with T>C).
Genotyping of the SNP was done using 5′exonuclease (Taqman®) chemistry on the ABI Prism® 7000 system (Applied Biosystems, Foster City, CA). All assays were validated by direct sequencing using Big Dye in 1.1 terminator chemistry (Applied Biosystems).
Statistical analyses
Summary statistics for categorical variables are shown as frequencies and percentages. Normally distributed continuous variables are shown as mean and SD or median and 5th–95th percentile range for variables with a non-normal distribution. Within all groups, the distributions of genotypes and consistency of genotype frequencies with the Hardy–Weinberg equilibrium were tested using the χ2 test on a contingency table of observed versus predicted genotype frequencies. The allelic frequencies were compared by the Fisher's exact test. Changes within groups were analyzed by the Wilcoxon signed-ranks test and differences between the two groups were analyzed by the Mann–Whitney U test. Correlations were analyzed by Spearman's rank order correlation coefficient. Predictive power of variables for binary end points was assessed using receiver operating characteristics (ROC) analysis.
The point estimates of the risk, the odds ratio (OR), and 95% confidence interval (CI) based on logistic regression were estimated and supplemented by their significance (Ward's test); univariate logistic regression was adopted for the preselection of variables for the multivariate model. Multivariate linear regression was applied for adjustment of markers of LV dysfunction on the influence of other variables. Preselection of variables based on univariate linear regression and a backward stepwise algorithm were used for the definition of these models.
A probability value of α<0.05 was considered significant. The power of tests and sample size were evaluated by EpiInfo6. Statistical analyses were undertaken using Stat View version 8.0 (SAS Institute Incorporated, Cary, NC), SPSS 18.0.1 (SPSS, Incorporated, Chicago, IL), and STATISTICA 8.0 (StatSoft, Incorporated, Tulsa, OK).
Results
Genotypes and levels of TIMP-1 were obtained for 556 patients of the study group. The gene for TIMP-1 is located on the X chromosome; men and women were therefore analyzed separately. Baseline characteristics for men and women are shown in Table 1.
Table 1.
Baseline Characteristics of Study Population
| All n=556 | Mena n=414 | Womenb n=142 | pb | |
|---|---|---|---|---|
| Age | 62.6 (44.6; 78.9) | 60.9 (43.9; 77.3) | 68.2 (51.8; 79.9) | <0.001 |
| Weight | 82 (63; 109) | 85 (69; 110) | 74 (53; 95) | <0.001 |
| BMI (kg/m2) | 27.7 (22.5; 34.8) | 27.8 (22.7; 35.4) | 27.1 (21.2; 35.2) | 0.083 |
| Systolic pressure (mmHg) | 140 (90; 185) | 140 (98; 185) | 140 (85; 190) | 0.967 |
| Diastolic pressure (mmHg) | 80 (55; 105) | 80 (60; 105) | 76 (50; 100) | 0.001 |
| Pulse pressure (mmHg) | 57 (27; 100) | 55 (30; 89) | 60 (25; 110) | 0.039 |
| Heart rate (min−1) | 73 (52; 107) | 72 (50; 110) | 74 (54; 105) | 0.656 |
| Time chest pain–balloon (min) | 170 (60; 590) | 169 (60; 585) | 180 (75; 600) | 0.022 |
| Acute heart failure | 27.7% | 26.1% | 32.2% | 0.152 |
| Smoking | 55.0% | 61.1% | 56.9% | 0.408 |
| Diabetes mellitus | 27.0% | 24.5% | 34.2% | 0.015 |
| Hypertension | 56.3% | 54.5% | 61.7% | 0.074 |
| Hyperlipoproteinemia | 83.0% | 80.4% | 89.3% | <0.001 |
| Previous ACEI/AT2 | 24.8/6.9% | 23.9/7.2% | 27.5/6.1% | 0.382 |
| Previous beta blockers | 26.6% | 24.5% | 32.9% | 0.031 |
| Previous statins | 16.5% | 15.3% | 20.1% | 0.108 |
| Previous diuretics | 16.7% | 13.7% | 25.5% | 0.001 |
| Glycaemia (mM) | 8.0 (5.5; 16.9) | 8.0 (5.4; 16.4) | 8.5 (5.6; 20.7) | 0.037 |
| Troponine I (ng/mL) | 49.5 (2.9; 183.1) | 51.6 (3.3; 198.0) | 43.8 (2.5; 146.9) | 0.018 |
| Creatinine (μM) | 88 (61; 137) | 90 (67; 133) | 80 (54; 144) | <0.001 |
| BNP admission (pg/mL) | 64 (15; 490) | 58 (15; 397) | 92 (15; 505) | <0.001 |
| BNP (24 h) (pg/mL) | 272 (68; 874) | 241 (61; 820) | 384 (89; 1305) | <0.001 |
| NTproBNP admission (pg/mL) | 220 (30; 4605) | 187 (27; 3004) | 405 (61; 6757) | <0.001 |
| NTproBNP (24 h)(pg/mL) | 1931 (370; 10427) | 1632 (343; 8099) | 3163 (707; 14711) | <0.001 |
| TIMP-1 admission (nM) | 109 (62; 257) | 107 (61; 252) | 118 (64; 268) | 0.022 |
| TIMP-1 (24 h)(nM) | 128 (77; 319) | 124 (74; 316) | 139 (88; 345) | 0.023 |
| TIMP-1 change after 24 h | 20 (−35; 135) | 19 (−35; 133) | 21.0 (−46; 137) | 0.994 |
| IRA–RIA | 43.2% | 42.9% | 44.3% | 0.750 |
| Previous instability | 34.6% | 33.6% | 37.6% | 0.213 |
| Previous infarction | 12.1% | 13.5% | 8.1% | 0.049 |
| Collaterals of IRA | 25.0% | 23.4% | 29.5% | 0.085 |
| EF (echo)(%) | 52 (30; 68) | 52 (29; 65) | 51 (30; 69) | 0.768 |
| EDV/BSA (mL/m2) | 61 (36; 92) | 62 (39; 93) | 56 (33; 85) | <0.001 |
| ESV/BSA (mL/m2) | 27 (14; 51) | 31 (16; 52) | 25 (12; 47) | 0.004 |
Significant values are indicated in bold.
Median (5th 95th percentile).
Mann–Whitney U test.
BMI, Body mass index; IRA, infarct-related artery; EDV, end-diastolic volume; ESV, end-systolic volume; BSA, body surface area.
TIMP-1 levels
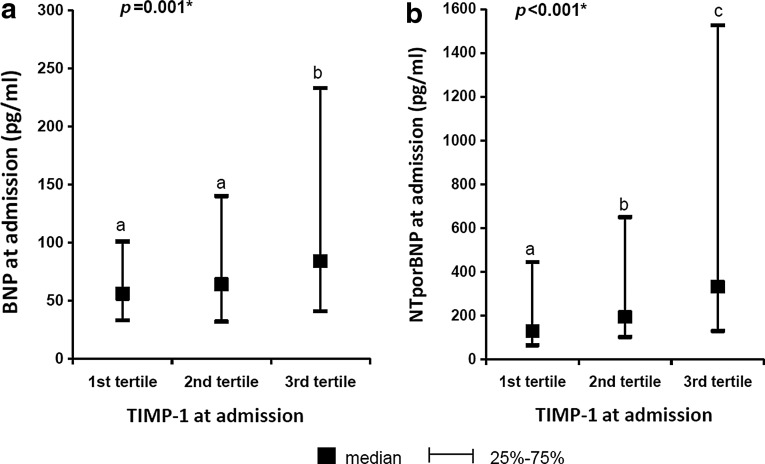
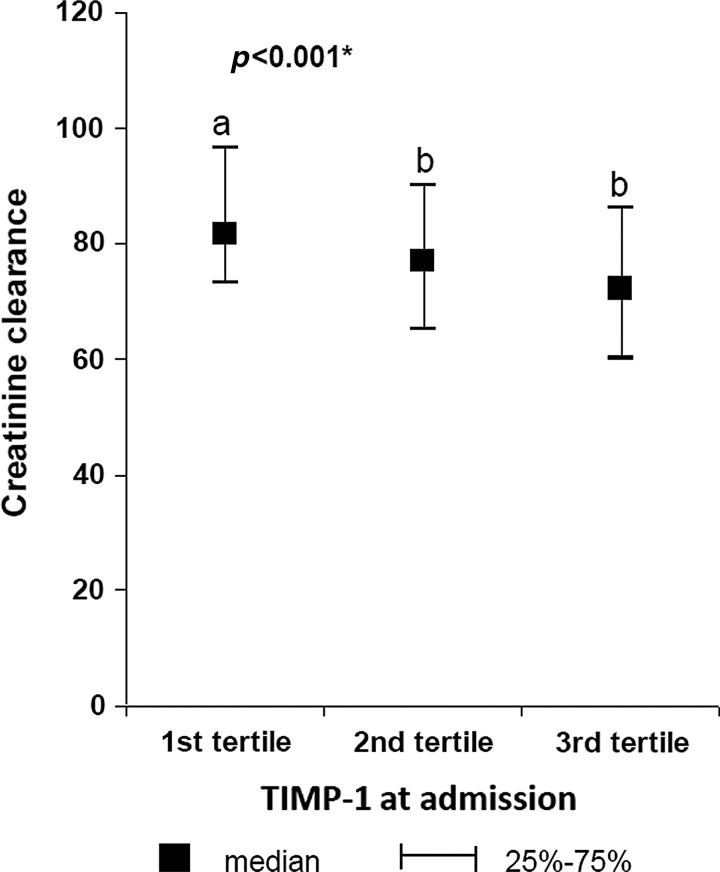
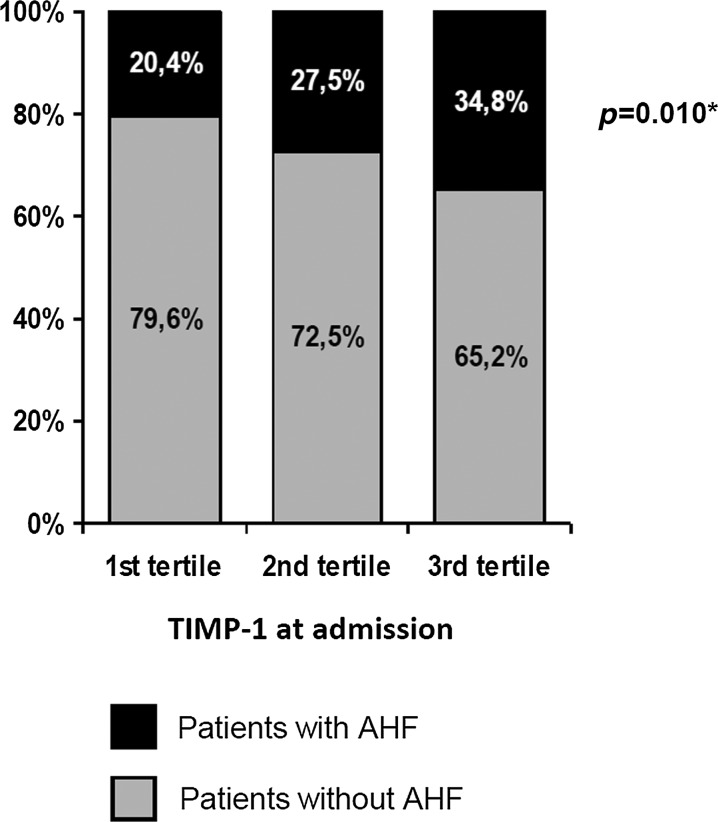
We found slightly higher levels of TIMP-1 for women in comparison with men (Table 1). The significant differences of TIMP-1 levels in different groups are shown in Table 2. Higher TIMP-1 levels were observed after 24 h after MI onset in comparison with the levels at admission. The significant differences in TIMP-1 levels at admission (TIMP-1/1) according to increasing levels of natriuretic peptides are demonstrated in Figure 1a and b and according to decreasing estimated creatinine clearance in Figure 2. Furthermore, growing proportion of patients with AHF according to increasing levels of TIMP-1 at admission was observed (Fig. 3). Factors affecting the levels of TIMP-1 at admission and at 24 h according to the multivariate linear regression for all patients are shown in Table 3.
Table 2.
Comparison of Tissue Inhibitors of Metalloproteinase-1 Levels in Different Groups of Patients According to Gender, Heart Failure, Ejection Fraction, and Diabetes Mellitus
| Characteristics of the groups | TIMP-1 group A (nM) | TIMP-1 group B (nM) | p |
|---|---|---|---|
| TIMP-1/1 men vs. women | 107.70 | 117.0 | 0.02 |
| TIMP-1/2 men vs. women | 125.0 | 139.0 | 0.02 |
| TIMP-1/1 vs. TIMP-1/2 for men | 107.0 | 125.0 | <0.01 |
| TIMP-1/1 vs. TIMP-1/2 for women | 117.7 | 139.0 | <0.01 |
| TIMP-1/1 for men with AHF vs. without AHF | 123.0 | 101.0 | <0.01 |
| TIMP-1/2 for men with AHF vs. without AHF | 145.0 | 118.6 | <0.01 |
| TIMP-1/2 for men with EF<40% vs. EF>40% | 138.0 | 121.0 | <0.01 |
| TIMP-1/2 for women with AHF vs. without AHF | 145.4 | 135.0 | 0.05 |
| TIMP-1/1 for men with DM vs. without DM | 118.0 | 101.2 | <0.01 |
| TIMP-1/2 for men with DM vs. without DM | 137.0 | 121.0 | <0.01 |
| TIMP-1/2 for women with DM vs. without DM | 145.9 | 134.2 | <0.01 |
TIMP-1/1–level of TIMP-1 at admission; TIMP-1/2 level of TIMP-1 at 24 h after MI onset; TIMP, tissue inhibitors of metalloproteinase; EF, left ventricular ejection fraction; AHF, acute heart failure; DM, diabetes mellitus.
FIG. 1.
(a,b) Patients were divided according to increasing values of tissue inhibitors of metalloproteinase (TIMP)-1 into 3 groups (tertiles); value of B-type natriuretic peptide (BNP) at admission and NT-proBNP increases with the value of TIMP-1.
FIG. 2.
Patients were divided according to increasing values of TIMP-1 into 3 groups (tertiles); the figure demonstrates decreasing creatinine clearance (estimated according to MDRD formula) in tertiles of patients with an increasing value of TIMP-1.
FIG. 3.
Figure 3 demonstrates the growing proportion of patients with acute heart failure in tertiles of patients with an increasing value of TIMP-1.
Table 3.
Multivariate Linear Analysis of Factors with Independent Relationship to Tissue Inhibitors of Metalloproteinase-1 Level at Admission and Tissue Inhibitors of Metalloproteinase-1 Level at 24 h for all Patients
| TIMP-1/1 | B (95% CI) | Beta | p |
|---|---|---|---|
| Sex: women | 0.075 (−0.010; 0.160) | 0.077 | 0.085 |
| Diabetes mellitus | 0.101 (0.023; 0.179) | 0.106 | 0.012 |
| NTproBNP at admissiona | 0.053 (0.029; 0.078) | 0.188 | 0.001 |
| Creatininea | 0.272 (0.120; 0.424) | 0.156 | 0.001 |
| TIMP-1/2 | |||
| Delaya | 0.090 (0.008; 0.173) | 0.096 | 0.033 |
| Diabetes mellitus | 0.097 (0.011; 0.183) | 0.097 | 0.028 |
| BB before hospitalization | −0.295 (−0.475; −0.116) | −0.168 | 0.001 |
| Statins before hospitalization | −0.218 (−0.397; −0.040) | −0.126 | 0.018 |
| Troponine Ia | 0.036 (0.006; 0.065) | 0.106 | 0.018 |
| Creatininea | 0.382 (0.221; 0.543) | 0.207 | 0.001 |
| BNP at admissiona | 0.045 (0.007; 0.083) | 0.106 | 0.020 |
| Thromboaspiration | −0.248 (−0.326; −0.170) | −0.271 | 0.001 |
Significant values are indicated in bold.
Logarithmic transformation.
Only variables were taken into multivariate analysis in which a significant relationship was found in univariate linear regression. All variables taken into univariate linear regression are listed in Table 1.
BNP, B-type natriuretic peptide.
TIMP-1 levels and LV function
Since we found higher levels of TIMP-1 at 24 h (TIMP-1/2) in males with LV dysfunction (EF<40%) (Table 2), multivariate logistic regression was performed and the level of TIMP-1/2 was identified as a factor with an independent relationship to left ventricle dysfunction (Table 4). According to ROC analysis, we identified cutoff values to predict EF<40% for TIMP-1 and compared area under the curve (AUC) for TIMP-1 with AUC of other biomarkers (Table 5). According to multivariate linear analysis, the TIMP-1 levels were not independent factors affecting left ventricle volumes EDV/BSA or ESV/BSA (data not shown).
Table 4.
The Multivariate Logistic Regression of Factors with an Independent Relationship to Acute Heart Failure and Ejection Fraction ≤40% for Males
| OR (95% CI) | p | |
|---|---|---|
| Acute heart failure | ||
| TIMP-1/2 | 5.570 (2.089; 14.850) | 0.001 |
| Pulse pressure | 0.967 (0.950; 0.984) | 0.001 |
| Glycaemiaa | 4.576 (1.761; 11.890) | 0.002 |
| IRA-RIA | 1.672 (0.895; 3.123) | 0.107 |
| Delaya | 1.726 (0.877; 3.398) | 0.114 |
| Collaterals of IRA | 0.441 (0.185; 1.055) | 0.066 |
| EF≤40% | ||
| TIMP-1/2 | 3.971 (1.355; 11.643) | 0.012 |
| Systolic pressure | 0.978 (0.960; 0.996) | 0.017 |
| Delaya | 3.048 (1.212; 7.664) | 0.018 |
| Glycaemiaa | 2.711 (0.726; 10.128) | 0.138 |
| IRA-LAD | 11.721 (4.019; 34.185) | 0.001 |
| Beta-Blockers | 2.201 (0.803; 6.036) | 0.125 |
| DCV | 1.991 (1.143; 3.467) | 0.015 |
| Hyperlipoproteinemia | 0.306 (0.112; 0.832) | 0.020 |
| Previous infarction | 6.239 (1.794; 21.703) | 0.004 |
Significant values are indicated in bold.
Only variables were taken into multivariate analysis in which a significant relationship was found in univariate logistic regression. All variables taken into univariate logistic regression are listed in Table 1; natriuretic peptides were not included into the multivariate model.
Logarithmic transformation.
TIMP-1/2–level of TIMP-1 at 24 h after MI onset; IRA, infarct-related artery; DCV, diseased coronary vessels; Delay, time chest pain–percutaneous coronary intervention.
Table 5.
Receiver Operating Characteristics Analysis for Prediction of Left Ventricular Ejection Fraction <40%
| AUC (95% CI)a | Sig. | Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| TIMP-1 (nM) at admission | 0.534(0.467; 0.601) | 0.357 | ≥78.70 | 94.4% | 17.6% |
| TIMP-1 (nM) 24 h | 0.616(0.538; 0.694) | 0.005b | ≥144.95 | 53.4% | 70.4% |
| Troponin I (ng/mL) | 0.700(0.630; 0.771) | <0.001b | ≥53.95 | 76.1% | 58.1% |
| BNP (pg/mL) at admission | 0.728(0.668; 0.788) | <0.001b | ≥79.80 | 76.4% | 64.5% |
| BNP (pg/mL) 24 h | 0.769(0.710; 0.827) | <0.001b | ≥299.70 | 80.3% | 62.2% |
AUC, area under the curve with 95% confidence interval (CI).
Significant p values.
TIMP, tissue inhibitors of metalloproteinase.
TIMP-1 levels and AHF
In total, 27.1% of patients had signs of AHF. Higher levels of TIMP-1 were determined in patients with AHF (Table 2). According to the multivariate logistic regression model, TIMP-1/2 was a factor with an independent relationship to AHF (Table 4). Figure 3 demonstrates association of TIMP-1 levels upon admission with AHF prevalence.
TIMP-1 levels and TIMP-1 polymorphism
No significant difference was found in TIMP-1 levels upon hospital admission and at 24 h after MI onset between the T allele group and the C allele group for males, and among the TT genotype group, the CT genotype group, and the CC genotype group for females. The univariate analysis did not reveal an association between levels of TIMP-1 and TIMP-1 polymorphism.
TIMP-1 polymorphism and LV function
In comparison with the C allele group of males, the T allele group had higher EF according to echocardiography (median EF 54.0% vs. 50.0%; p=0.042). However, after adjustment of EF for parameters affecting its value according to multivariate linear analysis (previous MI, Infarct related artery-LAD, troponin, pulse pressure, and NT-proBNP), the values of EF were comparable (50.2% vs. 50.2%; p=ns).
Discussion
The present work is the largest study evaluating the association between TIMP-1 levels and LV dysfunction as well as their relationship with rs4898 TIMP-1 polymorphism. We consider very important that it was carried out with a homogenous group of patients with STEMI treated with primary PCI, while a previous study evaluated both patients with STEMI treated with thrombolytic therapy and patients with non-STEMI.
Although slightly higher values of TIMP-1 at admission and at 24 h were found in the female group in comparison with males, according to the multivariate linear analysis (Table 3), the gender was not an independent factor influencing the TIMP-1 value. TIMP-1 is located at the X chromosome and should be expressed only from the active one. The hypothesis about higher levels of TIMP-1 because of expression from the inactive X chromosome in human females (Anderson and Brown, 2005) cannot be confirmed in the acute phase of MI according to our results.
We found higher levels of serum TIMP-1 at 24 h from the onset of MI compared with levels upon hospital admission. These results are in consensus with Webb et al. (2006) who demonstrated higher TIMP-1 levels at day 1 in post-MI patients, which remained elevated through day 180.
We found an association between higher TIMP-1 levels 24 h after MI onset and a worse EF. Similar findings of a negative correlation between the TIMP-1 level and EF and LVESV according to prehospital-discharge echocardiography were reported by Kelly et al. (2008). Also, Nilsson et al. (2012) reported a negative correlation between the TIMP-1 level at 24 h and EF according to the cardiac magnetic resonance assessed at day 5 and at 4 months. Weir et al. (2011) did not find a relationship between plasma concentrations of TIMP-1 and long-term remodeling of LV after MI. On the other hand, experimental knockout of the TIMP-1 gene in mice with MI caused worse LV function and a greater cardiac dilatation (Ikonomidis et al., 2005), and overexpression of TIMP-1 led to inhibition of MMP activity together with more preserved systolic and diastolic cardiac function (Jayasankar et al., 2004). Also postmyocardial pharmacologic inhibition of MMP was associated with an increased LVEF and reduced LVEDV (Ikonomidis et al., 2005). Unfortunately, our or other clinical studies failed to demonstrate that patients should profit from higher levels of TIMP-1. The main reason probably is that the value of TIMP-1 in clinical conditions is correlated with the infarction size [assessed via peak of creatin kinase (Kelly et al., 2008) or troponine (Table 3)]. Moreover, elevation of TIMP-1 over 24 h may be triggered by reperfusion injury of myocardium after PCI.
In clinical practice, the value of TIMP-1 at admission or after 24 h is not a useful biomarker for prediction of left ventricle EF; AUC for TIMP-1 is lower compared with Troponin T or BNP.
According to multivariate logistic regression, we demonstrated a relationship between elevated TIMP-1 levels and AHF (Table 4). In this analysis, the TIMP-1 value probably expresses (or correlates) the total extent of acute MI damage leading to AHF.
Observed elevation of TIMP-1 levels in patients with DM is in consensus with the literature and could reflect a compensatory response to degradative inflammatory enzymes (West et al., 2008; Kelly et al., 2010).
Association of TIMP-1 and creatinine levels is also in agreement with the literature as the elevation of TIMP-1 concentrations in serum and urine has been shown in patients with type-2 DM with worsening glomerular function and diabetic nephropathy (Kanauchi M et al., 1996).
We did not find an association of TIMP-1 polymorphism with circulating levels of TIMP-1 upon hospital admission or 24 h after MI onset, and no relationship between TIMP-1 polymorphism and the onset of LV dysfunction after acute MI. There are no data in the literature concerning the association between the exon 5 polymorphism and circulating levels of TIMP-1 in MI.
Very interesting information is that TIMP-1 levels are affected by previous treatment with statins and beta-blockers. It was demonstrated that statins stimulated TIMP-1, which subsequently inhibited MMP-1, -3, -9, and -13 (López-Cuenca et al., 2010). For the first time, we demonstrated that thrombus aspiration during acute STEMI could lead to lower circulating TIMP-1 levels. Improvement of microcirculation with possible lower myocardial injury after thrombus aspiration could be the mechanism responsible for this result.
For the first time, we evaluated TIMP-1 polymorphism in patients with STEMI and we did not find an association among TIMP-1 polymorphism and TIMP-1 levels. Although this result is enough to prove no influence of TIMP-1 polymorphism through TIMP-1 levels on AHF or LV dysfunction, we performed analysis and clearly confirmed no relationship of TIMP-1 polymorphism and LVEF or AHF in patients with MI. The results lead to a conclusion that the determination of TIMP-1 gene polymorphism of the exon 5 is not a useful laboratory test in clinical practice. We confirmed the relationship between TIMP-1 levels and LV dysfunction expressed by EF, but the value of TIMP-1 is not suitable in practice for predicting ventricular dysfunction. Rather than a causal relationship between the level of TIMP-1 and LV dysfunction, we consider this association as epiphenomena, as elevated TIMP-1 levels are given by the extent of MI. We demonstrated a relationship between TIMP-1 levels and AHF for the first time.
Study limitations
A relatively small number of women is the main limitation of the study. Another limitation could be the absence of other mutations in the noncoding and coding regions of the TIMP-1 gene.
Conclusion
The present study provides evidence that higher level of circulating TIMP-1 is independently associated with worse LV function and with AHF after STEMI treated by primary PCI. We did not find significant effects of TIMP-1 polymorphism on the TIMP-1 level, LV dysfunction, or AHF failure in patients with acute MI. Although previous studies demonstrated that TIMP-1 may play an important role in LV remodeling after MI, according to our results, the measurement of TIMP-1 level as a biomarker for early prediction of LV dysfunction has no value in clinical practice.
Acknowledgments
The study was supported by a grant from the Internal Grant Agency of Ministry of Health IGA NS 9894-4 and by European Regional Development Fund –Project of International Clinical Research Center, St. Anne's University Hospital Brno, Czech Republic (No.CZ.1.05/1.1.00/02.0123). We thank the study investigators for their contribution to the study.
University Hospital Brno, Czech Republic: Ludmila Dostalova, Katarina Marcinechova, Jana Gottwaldova, Ivana Klabenesova, Ludmila Malaskova, Roman Miklik; Masaryk University Brno, Czech Republic: Marie Tomandlova
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson CL. Brown CJ. Epigenetic predisposition to expression of TIMP1 from the human inactive X chromosome. BMC Genetics. 2005;6:48. doi: 10.1186/1471-2156-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicu CF. Stroud JD. Livesay VA, et al. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–H132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- Docherty AJP. Lyons A. Smith BJ, et al. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985;318:66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Furenes EB. Arnesen H. Solheim S, et al. The profile of circulating metalloproteinases after PCI in patients with acute myocardial infarction or stable angina. Thromb Res. 2009;124:560–564. doi: 10.1016/j.thromres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Ruoslahti E. Integrin Signaling. Science. 1999;285:1028–1033. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Hinterseher I. Krex D. Kuhlisch E, et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphisms in a caucasian population with abdominal aortic aneurysm. World J Surg. 2007;31:2248–2254. doi: 10.1007/s00268-007-9209-x. [DOI] [PubMed] [Google Scholar]
- Ikonomidis JS. Hendrick JW. Parkhurst AM, et al. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288:H149–H158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- Jayasankar V. Woo YJ. Bish LT, et al. Inhibition of matrix metalloproteinase activity by TIMP-1 gene transfer effectively treats ischemic cardiomyopathy. Circulation. 2004;110:180–186. doi: 10.1161/01.CIR.0000138946.29375.49. [DOI] [PubMed] [Google Scholar]
- Kanauchi M. Nishioka H. Nakashima Y, et al. Role of tissue inhibitors of metalloproteinase in diabetic nephropathy. Nippon Jinzo Gakki Shi. 1996;38:124–128. [PubMed] [Google Scholar]
- Kelly D. Khan SQ. Thompson M, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–2124. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. Squire IB. Khan SQ, et al. Usefulness of plasma tissue inhibitors of metalloproteinases as markers of prognosis after acute myocardial infarction. Am J Cardiol. 2010;106:477–482. doi: 10.1016/j.amjcard.2010.03.060. [DOI] [PubMed] [Google Scholar]
- López-Cuenca Á. Marín F. Roldán V, et al. Effects of atorvastatin 80 mg daily on indices of matrix remodelling in “high-risk” patients with ischemic heart disease. Int J Cardiol. 2010;139:95–97. doi: 10.1016/j.ijcard.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Nagase H. Woessner JF. Matrix Metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nilsson L. Hallén J. Atar D, et al. Early measurements of plasma matrix metalloproteinase-2 predict infarct size and ventricular dysfunction in ST-elevation myocardial infarction. Heart. 2012;98:31–36. doi: 10.1136/heartjnl-2011-300079. [DOI] [PubMed] [Google Scholar]
- Tyagi SC. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem. 1993;26:191–198. doi: 10.1016/0009-9120(93)90025-2. [DOI] [PubMed] [Google Scholar]
- Webb CS. Bonnema DD. Ahmed SH, et al. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- Weir RAP. Clements S. Steedman T, et al. Plasma TIMP-4 predicts left ventricular remodeling after acute myocardial infarction. J Card Fail. 2011;17:465–471. doi: 10.1016/j.cardfail.2011.02.002. [DOI] [PubMed] [Google Scholar]
- West MJ. Nestel PJ. Kirby AC, et al. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J. 2008;29:923–931. doi: 10.1093/eurheartj/ehn007. [DOI] [PubMed] [Google Scholar]