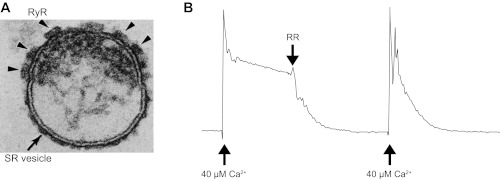
Fig. 1.
Characteristics of sarcoplasmic reticulum (SR) microsomes utilized for coupled ryanodine receptors isoform 1 (RyR1) gating studies. A: electron micrograph (250 × 200 nm) of a terminal cisternae vesicle of skeletal muscle SR. Cross section shows an isolated terminal cisternae of SR vesicle, typical of the field of SR R4 fractions. Junctional face membrane (the upper membrane) contains the foot structures (arrowheads) that have been identified as the RyR1. Tangential section of the junctional face membrane (not shown) reveals that the foot structures are oriented in a 2-dimensional interconnected quasi-checkerboard array (20–23, 58, 59). B: net Ca2+ loading rate of SR was measured by ultraviolet spectrometry using antipyrylazo III as described previously (3, 13). Fifty micrograms of SR microsomes were added to 1 ml of buffer containing 100 mM K-phosphate, 2 mM MgCl2, 1 mM ATP, and 0.5 mM antipyrylazo III to monitor Ca2+ levels as the increase in absorbance between 710 and 790 nm (3, 13). The total recording time was 8 min. After CaCl2 was increased to 40 μM, the Ca2+ uptake rate calculated in this example was 0.204 μmol Ca2+·min−1·mg protein−1 (control conditions). Addition of the RyR1 blocker ruthenium red (RR; 5 μM), increased the rate of loading to 1.28 μmol Ca2+·min−1·mg protein−1, indicating that most of the leak was mediated by RyR1 channels. In general, the R4 fractions used here had 15–20 pmol [3H]ryanodine binding per mg protein. Loading rates of the R4 fractions were >1 μmol Ca2+ min−1 mg protein−1 in the presence of RR and their loading ratio (absence of RR/presence of RR) was lower than 0.25. The numbers indicate a significant level of intact vesicles (that can uptake Ca2+) and a high density of RyR1 channels (that produce the leak).