Abstract
The thick ascending limb of the loop of Henle (TAL) reabsorbs ∼30% of filtered NaCl but is impermeable to water. The observation that little water traverses the TAL indicates an absence of water channels at the apical membrane. Yet TAL cells swell when peritubular osmolality decreases indicating that water channels must be present in the basolateral side. Consequently, we hypothesized that the water channel aquaporin-1 (AQP1) facilitates water flux across the basolateral membrane of TALs. Western blotting revealed AQP1 expression in microdissected rat and mouse TALs. Double immunofluorescence showed that 95 ± 2% of tubules positive for the TAL-specific marker Tamm-Horsfall protein were also positive for AQP1 (n = 6). RT-PCR was used to demonstrate presence of AQP1 mRNA and the TAL-specific marker NKCC2 in microdissected TALs. Cell surface biotinylation assays showed that 23 ± 3% of the total pool of AQP1 was present at the TAL basolateral membrane (n = 7). To assess the functional importance of AQP1 in the basolateral membrane, we measured the rate of cell swelling initiated by decreasing peritubular osmolality as an indicator of water flux in microdissected TALs. Water flux was decreased by ∼50% in Aqp1 knockout mice compared with wild-types (4.0 ± 0.8 vs. 8.9 ± 1.7 fluorescent U/s, P < 0.02; n = 7). Furthermore, arginine vasopressin increased TAL AQP1 expression by 135 ± 17% (glycosylated) and 41 ± 11% (nonglycosylated; P < 0.01; n =5). We conclude that 1) the TAL expresses AQP1, 2) ∼23% of the total pool of AQP1 is localized to the basolateral membrane, 3) AQP1 mediates a significant portion of basolateral water flux, and 4) AQP1 is upregulated in TALs of rats infused with dDAVP. AQP1 could play an important role in regulation of TAL cell volume during changes in interstitial osmolality, such as during a high-salt diet or water deprivation.
Keywords: cell volume, loop of Henle, water channels, vasopressin, AQP-1 knockout
the thick ascending limb of the loop of Henle (TAL) reabsorbs ∼30% of the filtered NaCl load. It is considered the “water-impermeant” segment of the nephron because it reabsorbs Na but not water, thereby diluting the forming urine and establishing the corticomedullary gradient necessary for water reabsorption by the collecting duct. Based on measurements of transepithelial (lumen-bath) water permeability, net water flux is relatively low in isolated, perfused TALs, even in the presence of arginine vasopressin (44). Thus, it has long been assumed that the TAL does not contain water channels. While this may be true of the apical membrane, changes in basolateral osmolality result in rapid changes in cell volume (18–20) strongly suggesting that water channels are present at the peritubular side of TAL cells to facilitate water flux. However, to our knowledge, the identity of these channels has never been studied.
Aquaporins (AQPs) are a family of transmembrane proteins that form water channels at the plasma membrane of many cells. Thirteen isoforms have been identified thus far, from AQP0 to AQP12 (2, 51). In the kidney, distribution of AQP isoforms appears to depend on the cell type (1). AQP2, 3, and 4 are the most abundant in the inner medulla and play an important role in water reabsorption by the collecting duct (28), whereas AQP1 appears to be most common in other areas of the kidney (34, 37, 45). In the cortex, AQP1 is constitutively expressed in the proximal tubule, where it plays a major role in water reabsorption (47). In the outer medulla, AQP1 is expressed in the descending thin limbs and vasa recta where it is necessary for concentrating the forming urine (11, 33, 37). Yet even though TALs make up at least 90% of the outer medulla, AQP1 expression has never been demonstrated in the TAL. Therefore, we hypothesized that AQP1 is expressed in the TAL where it facilitates water flux across the basolateral membrane.
MATERIALS AND METHODS
Animals.
Sprague-Dawley rats were purchased from Charles River (Kalamazoo, MI) and C57Bl 6J [wild-type (WT)] mice from Jackson Laboratories (Bar Harbor, ME), whereas Aqp1 −/− mice were kindly provided by Dr. Heddwen Brooks (University of Arizona) and were backcrossed to C57Bl 6J for at least six generations. Animals were fed a diet containing 0.22% Na and 1.1% K (Purina, Richmond, IN) for at least 7 days. On the day of the experiment, they were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). All protocols were approved and carried out in accord with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System.
Medullary TAL suspensions.
TAL suspensions were obtained from rats weighing 150–200 g and mice weighing 18–24 g as previously reported (24). Our working buffer (solution A) contained (in mmol/l) 130 NaCl, 2.5 NaH2PO4, 4 KCl, 1.2 MgSO4, 6 alanine, 1 Na2 citrate, 5.5 glucose, 2 Ca (lactate)2, and 10 HEPES (pH 7.4). After anesthesia, the kidneys were flushed with 40 ml ice-cold 0.1% collagenase in solution A gassed with compressed air via retrograde perfusion of the aorta. Coronal slices were cut from the kidneys and the inner stripe of the outer medulla was minced into 1-mm3 fragments at 4°C and digested in 0.1 mg/ml collagenase at 37°C for 30 min, gently agitating and gassing the tissue during each 5-min period. Finally, the tissue was filtered through a 250-μm nylon mesh and rinsed twice with the same solution, yielding a 92% pure TAL suspension, as previously reported (23).
Microdissection of TALs.
Rats weighing 100–150 g were anesthetized; the abdominal cavity was opened and the left kidney was bathed in ice-cold saline and removed. Coronal slices were placed in dissecting solution (solution A) and TALs were microdissected from the medullary rays under a stereomicroscope at 4–10°C (8–10).
Western blot.
Tubules were lysed by vortexing in a buffer containing 20 mmol/l HEPES (pH 7.4), 2 mmol/l EDTA, 300 mmol/l sucrose, 1.0% NP-40, 0.1% SDS, 5 μg/ml antipain, 10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mmol/l benzamidine, 5 μg/ml chymostatin, 5 μg/ml pepstatin A, and 0.105 mol/l 4(2-aminoethyl)-benzene sulfonyl fluoride (Sigma, St. Louis, MO). Samples were centrifuged at 6,000 g for 5 min at 4°C, measuring protein content in the supernatant when necessary. When microdissected tubules were used, 55 μl of buffer were added to the tubules and 50 μl of supernatant were loaded onto a 12% SDS-polyacrylamide gel; proteins were separated by electrophoresis and transferred to a PVDF membrane (Millipore, Bedford, MA). The membranes were incubated in blocking buffer containing 20 mmol/l Tris, 137 mmol/l NaCl, 5% nonfat dried milk, and 0.1% Tween-20 for 60 min and then with the following primary antibodies: antibody 1: rabbit anti-rat AQP1-specific polyclonal antibody (Alpha Diagnostics, San Antonio, TX, final concentration 5 ng/μl); antibody 2: rabbit anti-human AQP1-specific polyclonal antibody (EMD Biosciences, San Diego, CA; 1:1,000 dilution); or a 1:500 dilution of mouse nitric oxide synthase (NOS)3-specific polyclonal antibody (BD Biosciences) in blocking buffer for 1 h at room temperature. The membrane was washed in a buffer containing 20 mmol/l Tris, 137 mmol/l NaCl, and 0.1% Tween-20 and incubated with a secondary antibody against the appropriate IgG conjugated to horseradish peroxidase (Amersham Pharmacia Biotech, Arlington Heights, IL; 1:2,000 for AQP1 and 1:500 for NOS3). Finally, the membrane was exposed to Fuji RX film and the reaction products were detected with a chemiluminescence kit from Amersham Pharmacia Biotech.
Immunodetection of AQP1 and Tamm-Horsfall protein in paraformaldehyde-fixed, paraffin-embedded kidney sections using confocal microscopy.
Fixed, paraffin-embedded kidney slices from C57BL/6J and Aqp1 −/− mice were first deparaffinized by rinsing twice in xylene, hydrating gradually through 100, 95, and 70% ethanol and finally distilled water for 5 min each time. Slides were airdried and washed with PBS (150 mM NaCl and 10 mM Na2HPO4, pH 7.4) for 5 min, and then treated with 1% SDS in PBS for 5 min. Samples were blocked with 5% BSA in PBS, pH 7.4 for 30 min, and then incubated overnight (total: 19 h) with a mouse anti-AQP1 monoclonal antibody diluted in 5% BSA in PBS (AbD Serotec). The antibody was washed twice for 1 min and twice for 5 min, followed by incubation with a goat anti-Tamm-Horsfall polyclonal antibody diluted 1:100 in 5% BSA in PBS for 2 h (goat anti-human uromucoid, MP Biomedicals). The anti-Tamm-Horsfall antibody was washed with PBS twice for 1 min and twice for 5 min. Sections were incubated with fluorophore-conjugated secondary antibodies diluted in 5% BSA in PBS for 2 h: 1:100 Alexa Fluor 568 donkey anti-mouse and Alexa Fluor 488 donkey anti-goat (Invitrogen). Secondary antibodies were washed out with PBS twice for 1 min and twice for 5 min, and finally the slices were mounted using Fluoromount-G (Southern Biotech).
Fluorescent images of immunolabeled sections were acquired using a laser-scanning confocal system (Visitech International) mounted on a Nikon TE2000 microscope with a ×40 immersion oil objective. A 491-nm laser at 12% intensity was used to excite Tamm-Horsfall protein (THP). Emitted fluorescence was measured using a 488-nm primary dichroic and a 500-nm Long Pass barrier filter. For AQP1, we first established background fluorescence levels by imaging sections from Aqp1 −/− mice using a 561-nm laser at 25% intensity for excitation and measuring emitted fluorescence using a 568-nm primary dichroic and a 590-nm Long Pass barrier filter. The confocal aperture was 100 μm and a total of 100 images was obtained. Tamm-Horsfall labeling in the outer medulla was used to identify TALs and score the percentage of AQP1 labeling.
RT-PCR.
TALs (∼5 mm) were microdissected in less than 30 min and transferred to an RNase-free microcentrifuge tube. RNA was isolated using a commercial kit (RNeasy Mini Kit, Qiagen, Valencia, CA) following the manufacturer's instructions and eluted in a final volume of 30 μl; 2.5 μl random primers were immediately added to the RNA and samples were incubated for 5 min at 70°C. RT was performed at 37°C for 1 h in a final volume of 62.5 μl [2.5 μl M-MLV reverse transcriptase, 12.5 μl M-MLV RT 5× reaction buffer, 12.5 μl dNTPs (10 mM each), and 2.5 μl RNAsin ribonuclease inhibitor]. RNA (10 μg) was used for vascular smooth muscle cells. All PCR reactions were performed in a total volume of 50 μl in the presence of (in mM) 0.2 dNTPs and 3 MgCl2 as well as 1 μM of each primer (see Table 1) and 0.5 U Go Taq Felxi polymerase (Promega, Madison, WI). Under these conditions, all primer cDNA amplifications were optimal. The reaction mixture was first denatured at 94°C for 5 min and DNA was amplified 35 times between 94°C (denaturation) for 1 min, 55/60/64°C (annealing for AQP1, the Na-K-2Cl cotransporter NKCC2, and smooth muscle α-actin, respectively) for 30 s, and 72°C (extension) for 1 min. Samples were incubated for 7 min at 72°C after the final cycle. Twenty-five-microliter aliquots of the products from each PCR reaction were size-fractionated by electrophoresis on a 2.5% agarose gel and stained with ethidium bromide. DNA bands were visualized with an ultraviolet transilluminator.
Table 1.
Nucleotide sequence of the RT-PCR primers
| Name | Sequence | Size | Location |
|---|---|---|---|
| AQP1 | 5′-CCCTCTTCgTCTTCATCAgC-3′ (sense) | 430 bp | 124–143 (exon 1) |
| 5′-CTgAgCCACCTAAgTCTCgg-3′ (antisense) | 9,174–9,193 (exon 2) | ||
| NKCC2 | 5′-ggCCTCATATgCgCTT-3′ (sense) | 601 bp | 36,609–36,624 (exon 14) |
| 5′-AgTgTTTggCTTCATTCTCC-3′ (antisense) | 50,627–50,646 (exon 19) | ||
| sm α-actin | 5′-GAT CAC CAT CGG GAA TGA ACG C-3′ (sense) | 389 bp | 9,386–9,407 (exon 7) |
| 5′-CTT AGA AGC ATT TGC GGT GGA C-3′ (antisense) | 12,553–12,574 (exon 9) |
AQP, aquaporin; NKCC2, Na-K-2Cl cotransporter; sm α-actin, smooth muscle α actin.
Surface biotinylation of medullary TAL suspensions.
Cell surface proteins were biotinylated in medullary TAL suspensions using a modification of Ortiz's method (39). Briefly, rat TAL suspensions were equilibrated at 37°C for 15 min in solution A, adding 95% O2-5% CO2 every 5 min. TALs were incubated with 0.75 ml chilled biotinylation solution containing the cell-impermeant reagent NHS-SS-biotin (Pierce, Rockford, IL) at a concentration of 0.9 mg/ml on a rocker platform at 4°C. After 15 min, 0.75 ml freshly prepared NHS-SS-biotin (0.9 mg/ml) was placed on top and samples were incubated for 15 min. After biotinylation, TALs were washed with a 100-mM glycine solution to remove excess NHS-SS-biotin. TALs were pelleted by centrifugation at 93 g for 2 min and lysed at 4°C in a buffer containing 150 mM NaCl, 50 mM HEPES (pH 7.4), 5 mM EDTA, 2% Triton X-100, 0.2% SDS, and protease inhibitors [10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mmol/l benzamidine, 5 μg/ml chymostatin, 5 μg/ml pepstatin A (Sigma)]. After centrifugation, protein content in the supernatant was measured and 50 μg lysate were incubated overnight with streptavidin-coated agarose beads (Pierce) 4°C. Beads were precipitated by centrifugation at 16,000 g for 1 min and the supernatant was used to repeat the procedure using fresh streptavidin-coated beads for 2 h. Beads were pooled and precipitated by centrifugation at 16,000 g for 5 min, saving the supernatant on ice (intracellular or nonbiotinylated fraction). After being washed, proteins were extracted from the beads by boiling for 5 min in 55 μl SDS-loading buffer containing 50 mM DTT and 10% β-mercaptoethanol to cleave the disulfide bridge in NHS-SS-biotin. After centrifugation at 16,000 g for 3 min, the supernatant was placed on ice; 55 μl of fresh SDS-loading buffer were added to the beads and the tubes were boiled again. After centrifugation, the supernatant was combined with that from the first extraction (biotinylated fraction: 110 μl).
To estimate the percentage of total AQP1 contained in the cell membrane, one-third of the biotinylated fraction containing surface proteins and one-tenth of the supernatant (∼5 μg total protein) containing intracellular nonbiotinylated proteins were loaded into the same 12% SDS-polyacrylamide gel, resolved, and AQP1 was measured by Western blot as described above using antibody 1. Optical densities of surface and intracellular AQP1 bands (glycosylated + nonglycosylated) were used to calculate total AQP1 and per cent surface AQP1 as follows: total AQP1 (OD) = (OD intracellular band × 10) + (OD surface band × 3) % AQP1 in the surface fraction = [(OD surface × 3) × 100]/OD total AQP1.
Measurement of basolateral water flux.
Freshly microdissected TALs were transferred to a temperature-regulated chamber designed for fluorescent live-cell imaging. Tubules were held in place by concentric glass pipettes and maintained at 37°C during the experiment. The flow rate of the bath was 1.8 ml/min (solution A). Tubules were loaded by adding 1 μM CellTrace Calcein Green (Invitrogen) to the bath for 7 min and washing them with solution A for 10 min. The dye was excited at 488 nm with an argon/krypton laser, and fluorescence emitted was measured using a laser-scanning confocal system (Visitech International) mounted on a Nikon TE2000 microscope. After baseline fluorescence was stabilized, the osmolality of the bath was rapidly decreased by ∼50 mosmol/kgH2O by exchanging it with a buffer similar to solution A except that osmolality was reduced to 240 mosmol/kgH2O by adjusting NaCl content (20). The decrease in fluorescence, representing the increase in cell volume caused by swelling, was measured once every 2 s for 60 s. Rates of water influx were calculated from the slope of the initial rate of decrease in fluorescence, expressing the results as fluorescence U/s.
Effect of dDAVP infusion on TAL AQP1 expression.
Rats weighing 230–260 g were implanted with osmotic minipumps (Alzet, Cupertino, CA) filled with the arginine vasopressin analog [deamino-Cys1, d-Arg8]-vasopressin acetate hydrate (dDAVP; 10 ng/μl, Sigma) at a rate of 0.5 μl/h to deliver 5 ng/h. Controls were infused with vehicle. Two days later, animals were placed in metabolic cages and urine was collected for 24 h. After infusion for 3 days, medullary TAL suspensions were obtained and AQP1 expression was measured by Western blot.
Statistics.
Data are reported as means ± SE. Significance was tested by one-sample t-test or paired t-test as appropriate. All statistical analyses were performed by the Biostatistics Department of Henry Ford Hospital, taking P < 0.05 as significant.
RESULTS
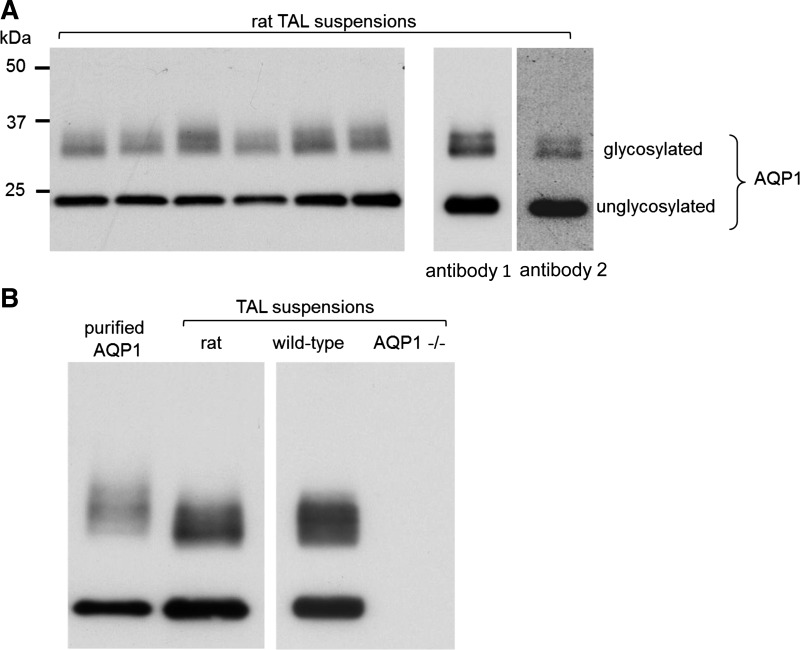
To investigate the hypothesis that AQP1 is expressed in the TAL, we first probed homogenates of medullary TAL suspensions with antibody 1 and detected bands characteristic of AQP1 protein on Western blot. As shown in Fig. 1A, two diffuse bands were detected: one of 34–40 kDa corresponding to the glycosylated form and a sharper 26-kDa band suggestive of the nonglycosylated form (32) (n = 6). As a first attempt to confirm these results, we used a distinct antibody (antibody 2) finding identical bands (Fig. 1A, right). Since antibody 1 appeared to be more sensitive, it was used for the remaining Western blots. To make sure these antibodies were specific for AQP1, human-purified AQP1 (26) (Fig. 1B, left) and TALs from Aqp1 −/− mice (Fig. 1B, right) were used as positive and negative controls, respectively.
Fig. 1.
Detection of aquaporin (AQP)1 in rat thick ascending limb (TAL) suspensions using Western blots. A: 10-μg suspensions from different animals were loaded into each lane (n = 6); right: identical sized bands were obtained using 2 distinct anti-AQP1 antibodies. Here, the membrane was cut and each piece was used to test the different antibodies (brightness and contrast were enhanced in this particular blot to make the bands visible on the picture). B: purified AQP1 as a positive control; right: TALs from Aqp1 −/− mice as a negative control. WT, wild-type. Representative blots from different gels are separated by the white space between them.
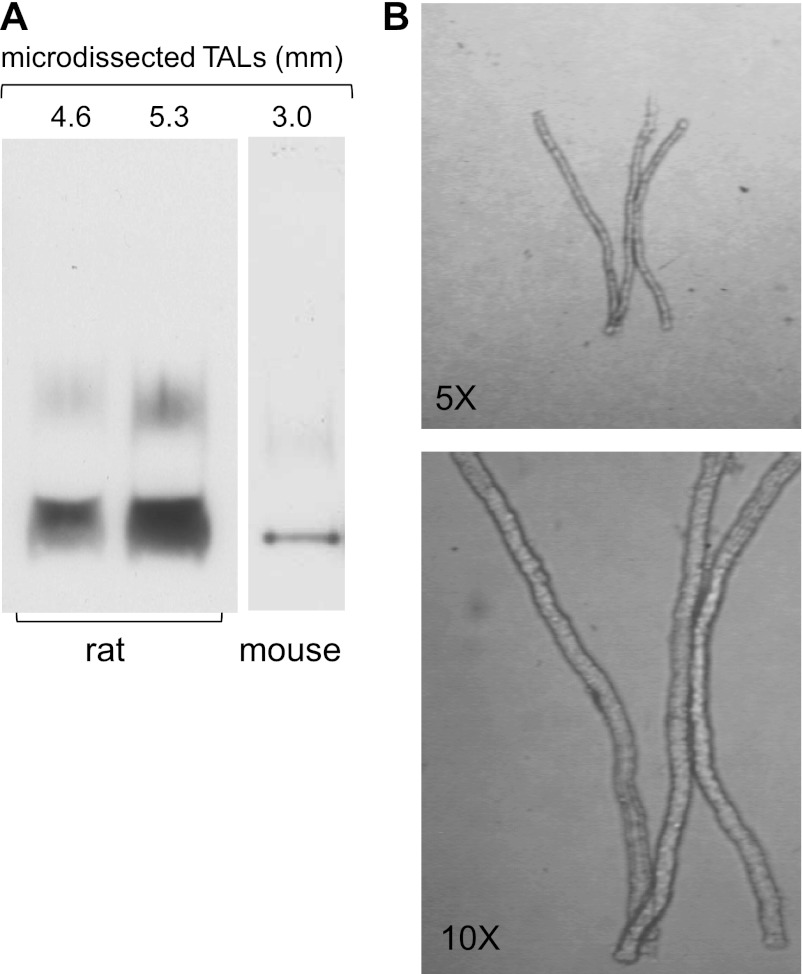
Although the outer medulla is composed primarily of TALs, we recognized that the suspensions might have contained small amounts of vasa recta and/or descending thin limbs, which could have accounted for the positive AQP1 signal. To account for this, we repeated the Western blots using microdissected TALs (4.2–5.5 mm) from both rats and mice and also found detectable levels of AQP1 expression (Fig. 2A; n = 5). These results are not due to other cell types remaining attached to the TAL since clean tubules are obtained by microdissection (Fig. 2B). This confirmed that 1) isolated rat and mouse TALs express AQP1 and 2) the signal did not originate from other structures within the renal medulla.
Fig. 2.
A: detection of AQP1 in microdissected rat and mouse TALs using Western blots. Isolated TALs from different animals were loaded into each lane. The numbers above the blot indicate the total length of the tubules. B: transmitted light images of microdissected rat TALs. Top: ×5 magnification. Bottom: ×10 magnification. Representative blots from different gels are separated by the white space between them.
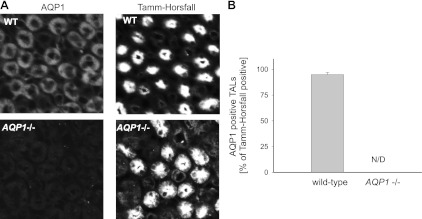
To further confirm the findings from the Western blot analysis, we investigated AQP1 expression in the TAL by double immunofluorescence and confocal microscopy of paraffin-fixed tissue slices taken of the outer medulla. We counterstained with an antibody against THP, a typical marker of TALs (3). All images showed tubular structures intensely labeled with the anti-Tamm-Horsfall antibody; however, AQP1-positive staining could only be detected in WT kidneys and not in kidneys from Aqp1 −/− mice (Fig. 3A). We counted all tubular structures labeled with the anti-Tamm-Horsfall antibody that were positive for AQP1. A total of 888 tubules from 6 independent tissue sections obtained from 3 different animals was scored, showing that in the kidneys of WT mice 95 ± 2% of TALs were positive for AQP1 (Fig. 3B).
Fig. 3.
A: double immunofluorescence labeling for AQP1 (left) and the luminal marker Tamm-Horsfall protein (right) in outer medullary slices from representative WT (top) and Aqp1 −/− mice (bottom; n = 6). B: quantitative data showing the percentage of Tamm-Horsfall-positive tubules also positive for AQP1 labeling in the outer medulla of WT and Aqp1 −/− mice (n = 6).
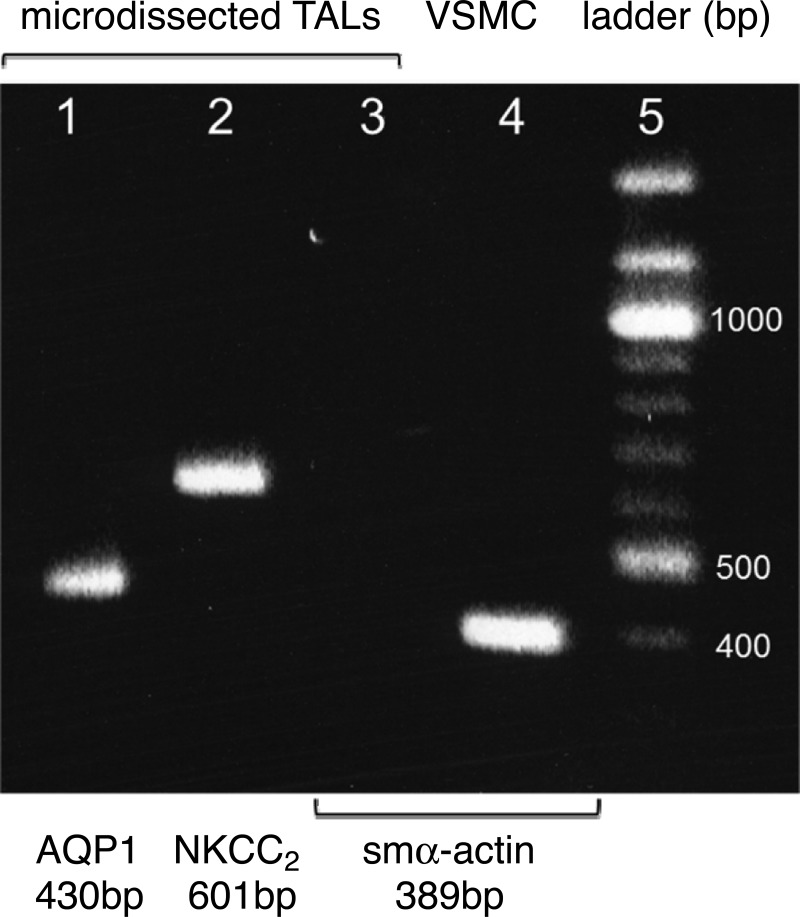
To show that AQP1 is synthesized in the TAL and not simply taken up or sticking to TAL membranes, we measured mRNA by RT-PCR. Analysis of microdissected TALs (3.8–5.8 mm) showed a 430-bp cDNA corresponding to AQP1 mRNA. Parallel experiments using specific primers for the Na-K-2Cl cotransporter (NKCC2; a specific marker for the TAL) (53) and smooth muscle α-actin (a marker for vasa recta pericytes) (41) showed that all samples from microdissected TALs amplified products for NKCC2 (601 bp) but were negative for smooth muscle α-actin (389 bp), a marker for vasa recta. PCR reactions without adding either specific primers or the reverse-transcriptase enzyme were used as negative controls, whereas RNA isolated from primary cultures of rat vascular smooth muscle cells was utilized as a positive control for smooth muscle α-actin (n = 5; Fig. 4), confirming the proper functionality of the RT-PCR reaction. These data confirmed the presence of AQP1 RNA in the TAL and discarded a potential cross contamination with other medullary structures.
Fig. 4.
Representative agarose mini-gel showing the amplification DNA products obtained by PCR using the same RT reaction from microdissected rat TALs for all genes 1: AQP1; 2: Na-K-2Cl cotransporter (NKCC2; a specific marker for the TAL); 3: smooth muscle α actin (smα-actin; a specific marker for the vasa recta); 4: amplification product for smα-actin using RNA from vascular smooth muscle cells (VSMC) as a positive control (n = 5).
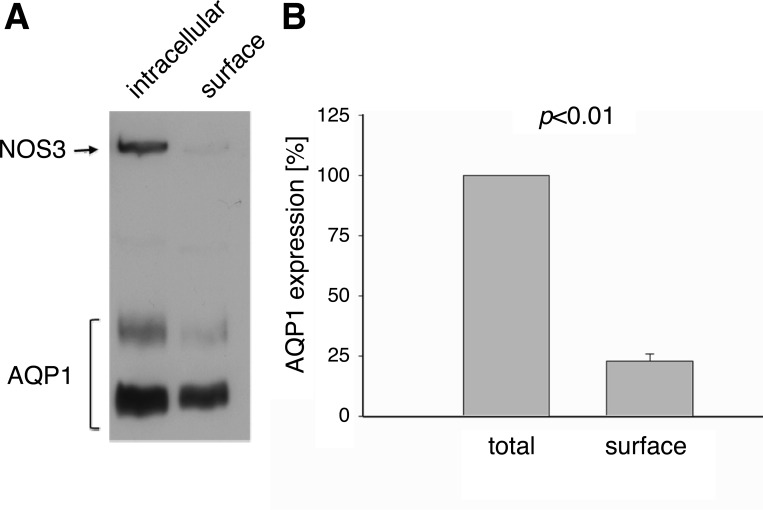
For water channels to be functional, they must be properly inserted at the plasma membrane. To determine cellular localization of AQP1 in the TAL, we measured membrane insertion of AQP1 using cell surface biotinylation of rat medullary TAL suspensions and found that under our experimental conditions, 23 ± 3% of the total pool of AQP1 (intracellular + extracellular) was present in the plasma membrane (n = 7; Fig. 5). When we employed the intracellular protein NOS3 as a control for purity of the surface fraction, we found that it was absent in the extracellular fraction, indicating that we were only labeling and pulling down membrane-associated proteins. In additional control experiments, we deduced that all biotinylated AQP1 was recovered after the second round of precipitation, as none was detected after a third round. We also established that all biotinylated AQP1 was eluted from the beads after being boiled twice in 50 mM DTT/β-mercaptoethanol (10%). These data confirmed the specificity of our detection system suggesting that AQP1 was inserted into the plasma membrane of TAL cells.
Fig. 5.
Cell-surface biotinylation of AQP1 in rat TAL suspensions. A: representative Western blot showing intracellular and surface AQP1. The intracellular protein nitric oxide synthase (NOS)3 is not present in the surface fraction. Note that different amounts of total protein were loaded for each fraction as indicated in materials and methods. B: mean data showing the percentage of the total pool of AQP1 that was inserted into the plasma TAL membrane (surface fraction; n = 7).
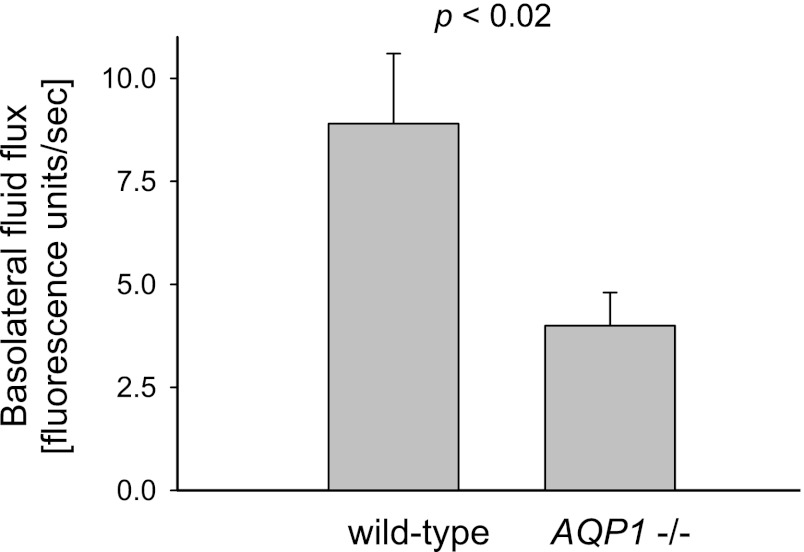
To determine whether membrane-associated AQP1 mediates water transport across the basolateral membrane of TALs, we calculated the initial rate of hypo-osmolality-induced cell swelling as a measure of basolateral water flux. We found that water influx was 8.9 ± 1.7 fluorescent units (fu)/s in TALs microdissected from WT mice (n = 6) but only 4.0 ± 0.8 fu/s or 50% slower in tubules from Aqp1 −/− mice (P < 0.02 vs. WT; n = 7; Fig. 6). These results show that AQP1 is at least partly responsible for mediating basolateral water permeability in TALs.
Fig. 6.
Rate of basolateral fluid flux initiated by decreasing extracellular osmolality in microdissected TALs from WT and Aqp1 −/− mice (n = 6–7).
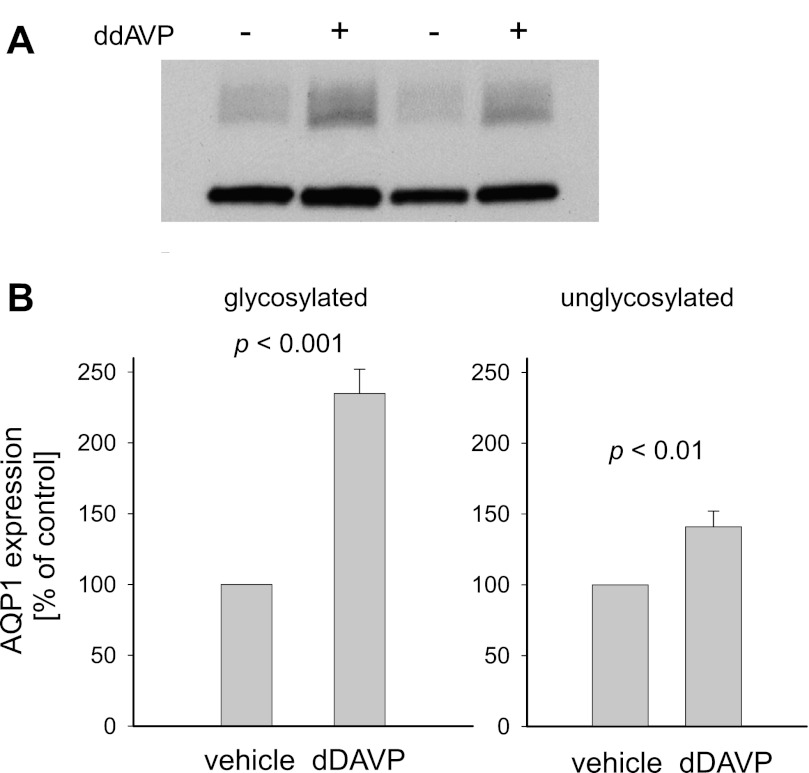
Finally, to evaluate the in vivo relevance of our previous observations, we tested whether AQP1 expression in the TAL is regulated by arginine vasopressin as it occurs with other aquaporins (5, 49). For this, we infused rats for 3 days with the vasopressin analog dDAVP. To ensure that dDAVP infusion enhanced kidney reabsorptive capacity, we measured urine volumes and osmolalities. Twenty-four-hour urine volume was 3.9 ± 0.4 ml/100 g in vehicle-infused rats but fell to 2.3 ± 0.1 ml/100 g in rats given dDAVP (P < 0.02 vs. vehicle; n = 4). In addition, urine osmolality was 1,214 ± 34 mosmol/kgH2O in the controls but 2,339 ± 87 mosmol/kgH2O in dDAVP-infused rats (P < 0.0001 vs. vehicle; n = 4). Importantly, when we measured AQP1 expression in TAL suspensions by Western blot, glycosylated AQP1 expression increased by 135 ± 17% in dDAVP-infused rats (P < 0.001 vs. vehicle; n = 4) while nonglycosylated AQP1 was enhanced by 41 ± 11% (P < 0.01 vs. vehicle; n = 4; Fig. 7). Thus, our data suggest TAL AQP1 expression can be physiologically regulated in response to arginine vasopressin.
Fig. 7.
Effect of chronic infusion of the arginine vasopressin analog [deamino-Cys1, d-Arg8]-vasopressin acetate hydrate (dDAVP) on AQP1 expression by rat TAL suspensions. A: representative Western blot of 2 different experiments. B: quantitative data showing the percent change in glycosylated (left) and nonglycosylated AQP1 (right) after dDAVP infusion (n = 4).
DISCUSSION
Although the existence of water channels at the basolateral side of TAL cells was evidenced by the original experiments done by the Hebert laboratory (19), to our knowledge our data are the first to demonstrate the presence of a specific aquaporin in the TAL. We found that 1) AQP1 protein is expressed in TAL suspensions as well as microdissected tubules from rats and mice, 2) AQP1 and NKCC2 mRNA are present in microdissected rat TALs, 3) AQP1 can be detected in 95% of TALs by double immunofluorescence, 4) ∼23% of the total AQP1 pool is inserted into the cell membrane of TALs, 5) the rate of water flux across the basolateral side of TALs was reduced by ∼50% in tubules isolated from Aqp1 −/− mice, and 6) TAL AQP1 expression is increased in rats with hyperosmolality caused by chronic infusion of dDAVP.
The first indication that AQP1 might be expressed in the TAL was finding the two characteristic bands for glycosylated and nonglycosylated AQP1 on Western blots of TAL homogenates. The specificity of the antibodies used was confirmed by using human purified AQP1 from red blood cells and tissue from Aqp1 −/− mice as positive and negative controls, respectively (26). Our antibodies clearly recognized both glycosylated and nonglycosylated forms of the purified AQP1 but there appeared to be a shift in the molecular weight of glycosylated AQP1 (Fig. 1B, left), possibly due to differing degrees of glycosylation between rat and human AQP1. When we used TALs isolated from mice, we found that AQP1 was only detected in WT TAL suspensions, not Aqp1 −/−, suggesting that the antibodies we used are specific for AQP1 and do not cross-react with other proteins of similar molecular weight.
Although our previous work established that our TAL suspensions are 92% pure (23), the outer medulla also contains structures that express AQP1 such as vasa recta and descending thin limbs (34, 37, 45). We therefore used the microdisection technique to isolate TALs and detected AQP1 expression. Utilizing this stringent isolation procedure, we discarded potential cross-contamination from other structures. In addition, by immunofluorescence, the majority of the outer medullary nephrons that stained positive for the TAL-specific luminal marker THP were also positive for AQP1. These experiments were done with an anti-AQP1 antibody that was distinct from the ones used for Western blotting and for which the specificity was tested in kidney sections from Aqp1 −/−. The immunofluorescence images suggest a diffuse distribution of AQP1 across the TAL cells; however, it is possible that a small fraction of AQP1 is inserted at the basolateral membrane while the majority of the AQP1 pool resides intracellularly as it occurs for other membrane-associated proteins in this segment (39). However, these experiments do not provide any information on subcellular localization because the technique used does not have a high enough resolution to distinguish between different cell compartments.
In addition to the protein data, we confirmed the presence of AQP1 mRNA in microdissected rat TALs. Using the same RT reaction, we found the amplification products for AQP1 and NKCC2 mRNA. NKCC2 is specifically expressed in the TAL and is not found in any other cells within the renal medulla (53). In contrast, the amplification product for smooth muscle α-actin (a vasa recta marker) was not found. These data indicate that AQP1 is synthetized by the TAL rather than resulting from residual membrane of neighboring structures sticking to the TALs after the microdissection procedure. Smooth muscle α-actin is a specific marker for vascular smooth muscle cells and thus served as a control for contamination with vasa recta (41). Under the same conditions, smooth muscle α-actin was detected using mRNA isolated from vascular smooth muscle cells, indicating that the conditions for RT-PCR were adequate to detect the mRNA of interest. Thus, AQP1 mRNA is located in the TAL and our findings cannot be attributed to the presence of vasa recta. Unfortunately, to our knowledge specific markers for descending thin limbs are not available; however, their presence is unlikely due to the nature of the microdissection technique (6, 7).
Because TAL cells rapidly shrink and/or swell in response to acute changes in basolateral osmolality (18–20), one would expect AQP1 to be inserted into the plasma membrane of TAL cells. Our cell surface biotinylation data indicate that a significant proportion of the total pool of AQP1 was inserted into the plasma membrane. Since the apical membrane of TAL cells is impermeable to water (20), any water channel must be located in the basolateral membrane only, which explains why even though water channels are expressed in the TAL this nephron segment exhibits very low lumen-bath flux. However, we recognize that by using TAL suspensions to perform cell surface biotinylation, we could be picking up AQP1 from other structures, since as we mentioned before, TAL suspensions are actually only ∼92% pure. Therefore, although we are confident that the contribution of cell surface-associated AQP1 from the vasa recta and descending thin limbs would be minimal, the percentage of AQP1 present at the cell membrane could have been overestimated and should be interpreted with caution.
Our measurements of basolateral water flux in TALs from Aqp1 −/− mice revealed a ∼50% reduction compared with controls, indicating that AQP1 facilitates about half of basolateral water flux across the basolateral membrane. That in turn suggests there might be other water channels in Aqp1 −/− TALs, although their identity is still unknown.
Since other aquaporins in the distal nephron are regulated by arginine vasopressin (5, 29, 49), we next asked whether this was also the case for AQP1 expression in the TAL. We found that in response to dDAVP infusion, TAL AQP1 expression increased by ∼135% (glycosylated form) and ∼41% (nonglycosylated form). As expected, these animals revealed a ∼92% increase in urine osmolality, which is a reflection of interstitial osmolality. Thus, one mechanism for the regulation of AQP1 expression in the TAL could be in response to changes in interstitial osmolality, which allows efficient regulation of cell volume.
In summary, our data indicate that AQP1 is only expressed in the basolateral side of the TAL cell, property that allows for cell volume regulation in response to changes in peritubular osmolality. The absence of water channels at the apical side of TAL cells is what prevents this nephron segment from reabsorbing water. The specific signaling events targeting AQP1 to the basolateral membrane only are unknown and they merit further investigation.
In contrast to our findings, other researchers have not detected AQP1 expression in the TAL. While the reason for the discrepant findings cannot be known with certainty, differences in experimental methodology may have contributed. For example, these studies utilized immunohistochemistry (37, 38, 45) and thus the conditions necessary to detect AQP1 in the TAL may not have been optimal. In this regard, the high abundance of AQP1 in the proximal tubule might have masked its presence in the TAL because there is less expression in this segment. In addition, most experiments examining AQP1 expression in the kidney were performed using an AQP1 antibody against a short amino acid peptide corresponding to the 10 amino terminus of human AQP1 (34, 37, 38, 48). In another report (45), whole purified human AQP1 was used to generate the antibody. But we used antibodies against a short COOH-terminus amino acid synthetic peptide corresponding to either rat or human AQP1, and we cannot overlook the possibility that depending on the type of cell involved, posttranslational modifications of AQP1 (such as protein-protein interactions) could prevent the antibody from recognizing the protein. Thus, different antibodies may provide variable results depending on which specific epitope is targeted.
Our finding that only a small fraction of the total pool of AQP1 was inserted into the plasma membrane raises the possibility for AQP1 trafficking between intracellular compartments and the plasma membrane, such that cell surface expression of AQP1 could be regulated by changing the rate of endocytosis and/or exocytosis as with other cells and other members of the AQP family (17, 27, 35, 36). In addition, it remains unclear why our data showed an approximately threefold difference in dDAVP-stimulated expression of glycosylated vs. nonglycosylated AQP1; however, it could be that glycosylation determines where AQP1 is located within cell compartments, as with other membrane-associated proteins (13, 21, 40). All these possibilities are beyond the scope of this manuscript but merit further investigation.
Our finding of augmented AQP1 expression in the TAL of dDAVP-infused rats suggested that AQP1 is under the control of physiologic regulation. Although potential mechanisms for the effect of dDAVP on AQP1 expression could be a direct effect of dDAVP, we cannot rule out this to be secondary to the increased interstitial osmolality as it has been reported for other AQPs in vitro and in vivo (29–31, 43). Our finding that AQP1 expression is enhanced in the TAL of dDAVP-infused rats appears to contradict a previous report by Terris et al. (49). In their study, it was found that dDAVP infusion did not change the level of AQP1 expression in the outer medulla of rats. At least two possibilities may explain the discrepant results. First, we measured AQP1 expression after 3 days of infusion while they performed the experiments after 5 days and using a higher dose of dDAVP. It is possible that the effect of dDAVP infusion on AQP1 expression is transient and dose-dependent with expression returning to basal levels at day 5 likely as part of the mechanism of renal escape from vasopressin-induced antidiuresis (16, 50). Second, elevated levels of AQP1 in the TAL could have been overlooked in their study due to the presence of vasa recta and descending thin limbs in their preparation which may be dDAVP-insensitive and express AQP1 at higher levels.
The precise role of AQP1 in the TAL remains unclear. The osmolality of the renal medulla varies daily depending on salt consumption, hydration status, and or renal perfusion pressure (15, 24, 46). Free osmosis across the cell membrane is not sufficient for rapid osmotic equilibration under these conditions and it has previously established that AQP channels are required to ensure appropriate membrane permeability to water molecules (4). The finding that most cells in the body express water channels supports the hypothesis that if cells are not able to shrink or swell in response to extracellular osmotic challenges, chronic exposure to these differences in tonicity could lead to compromised cell function, homeostasis, and survival. Thus, AQP1 could mediate the rapid changes in TAL cell volume during adaptations to the hyper- or hypoosmotic stress, thus protecting cells against cell damage and promoting survival. In line with this idea, a recent report by Conner et al. (12) demonstrated that rapid translocation of AQP1 regulates cellular water flow providing a mechanism for changes in water transport that are required in response to constantly changing extracellular water availability. In addition, we previously reported that AQP1 transports NO across cell membranes (22, 25, 26). Since the TAL produces NO, which exerts a paracrine action against other renomedullary structures (42, 52), it is worth investigating whether AQP1 facilitates diffusion of NO across the TAL cell membrane and into other medullary structures. Dickhout et al. (14) suggested that NO from the TAL diffuses to the adjacent vasa recta, causing relaxation, so that TAL-derived NO may help regulate medullary blood flow and therefore blood pressure. We are currently testing whether AQP1 facilitates diffusion of NO out of the TAL and into the vasa recta pericytes and whether this mechanism plays any physiological role in regulating medullary perfusion, sodium excretion, and hypertension.
GRANTS
This work was supported by a National Institutes of Health postdoctoral fellowship (DK081333-01) to M. Herrera and an American Heart Association postdoctoral fellowship (11POST7490010) to P. Cabral.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.D.C. and M.H. performed experiments; P.D.C. and M.H. analyzed data; P.D.C. and M.H. interpreted results of experiments; P.D.C. and M.H. prepared figures; P.D.C. and M.H. edited and revised manuscript; P.D.C. and M.H. approved final version of manuscript; M.H. conception and design of research; M.H. drafted manuscript.
REFERENCES
- 1. Agre P. Homer W. Smith award lecture. Aquaporin water channels in kidney. J Am Soc Nephrol 11: 764– 777, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel proteins. Am J Physiol Renal Fluid Electrolyte Physiol 265: F461, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531– 538, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Benga G, Popescu O, Borza V, Pop VI, Muresan A, Mocsy I, Brain A, Wrigglesworth JM. Water permeability in human erythrocytes: identification of membrane proteins involved in water transport. Eur J Cell Biol 41: 252– 262, 1986 [PubMed] [Google Scholar]
- 5. Brooks HL, Ageloff S, Kwon TH, Brandt W, Terris JM, Seth A, Michea L, Nielsen S, Fenton R, Knepper MA. cDNA array identification of genes regulated in rat renal medulla in response to vasopressin infusion. Am J Physiol Renal Physiol 284: F218– F228, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Burg M, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293– 1298, 1966 [DOI] [PubMed] [Google Scholar]
- 7. Burg MB. Perfusion of isolated renal tubules. Yale J Biol Med 45: 321– 326, 1972 [PMC free article] [PubMed] [Google Scholar]
- 8. Cabral PD, Garvin JL. Luminal flow regulates NO and O2− along the nephron. Am J Physiol Renal Physiol 300: F1047– F1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol 303: F194– F200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cabral PD, Hong NJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185– F1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chou CL, Knepper MA, Hoek AN, Brown D, Yang B, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J Clin Invest 103: 491– 496, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conner MT, Conner AC, Bland CE, Taylor LH, Brown JE, Parri HR, Bill RM. Rapid aquaporin translocation regulates cellular water flow: the mechanism of hypotonicity-induced subcellular localization of the aquaporin 1 water channel. J Biol Chem 287: 11516– 11525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deslauriers B, Ponce C, Lombard C, Larguier R, Bonnafous JC, Marie J. N-glycosylation requirements for the AT1a angiotensin II receptor delivery to the plasma membrane. Biochem J 339: 397– 405, 1999 [PMC free article] [PubMed] [Google Scholar]
- 14. Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487– 493, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Dobrowolski L, Badzynska B, Walkowska A, Sadowski J. Osmotic hypertonicity of the renal medulla during changes in renal perfusion pressure in the rat. J Physiol 508: 929– 935, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852– 1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gradilone SA, Carreras FI, Lehmann GL, Marinelli RA. Phosphoinositide 3-kinase is involved in the glucagon-induced translocation of aquaporin-8 to hepatocyte plasma membrane. Biol Cell 97: 831– 836, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. I. ADH dependency and nephron heterogeneity. Am J Physiol Cell Physiol 250: C907– C919, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl−-HCO3− exchange in basolateral membranes. Am J Physiol Cell Physiol 250: C920– C931, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Hebert SC, Sun A. Hypotonic cell volume regulation in mouse medullary thick ascending limb: effects of ADH. Am J Physiol Renal Fluid Electrolyte Physiol 255: F962– F969, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Hendriks G, Koudijs M, van Balkom BW, Oorschot V, Klumperman J, Deen PM, van der Sluijs P. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem 279: 2975– 2983, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Herrera M, Garvin JL. Aquaporins as gas channels. Pflügers Arch 462: 623– 630, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Herrera M, Garvin JL. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol Renal Physiol 287: F231– F235, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Herrera M, Garvin JL. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol 288: F58– F64, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292: F1443– F1451, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157– 164, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa Y, Yuan Z, Inoue N, Skowronski MT, Nakae Y, Shono M, Cho G, Yasui M, Agre P, Nielsen S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am J Physiol Cell Physiol 289: C1303– C1311, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, Mandon B, Chou CL, Kishore BK, Nielsen S. Renal aquaporins. Kidney Int 49: 1712– 1717, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Kortenoeven ML, Trimpert C, Brand MV, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB, but may involve Epac. Am J Physiol Renal Physiol 302: F1395– F1401, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Kortenoeven ML, van den Brand M, Wetzels JF, Deen PM. Hypotonicity-induced reduction of aquaporin-2 transcription in mpkCCD cells is independent of the tonicity responsive element, vasopressin, and cAMP. J Biol Chem 286: 13002– 13010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Wang W, Summer SN, Cadnapaphornchai MA, Falk S, Umenishi F, Schrier RW. Hyperosmolality in vivo upregulates aquaporin 2 water channel and Na-K-2Cl cotransporter in Brattleboro rats. J Am Soc Nephrol 17: 1657– 1664, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Ma T, Frigeri A, Tsai ST, Verbavatz JM, Verkman AS. Localization and functional analysis of CHIP28k water channels in stably transfected Chinese hamster ovary cells. J Biol Chem 268: 22756– 22764, 1993 [PubMed] [Google Scholar]
- 33. Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273: 4296– 4299, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Maeda Y, Smith BL, Agre P, Knepper MA. Quantification of aquaporin-CHIP water channel protein in microdissected renal tubules by fluorescence-based ELISA. J Clin Invest 95: 422– 428, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 276: G280– G286, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424– 429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1023– F1037, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371– 383, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608– F616, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+-K+-2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl− transport. Am J Physiol Renal Physiol 290: F1094– F1102, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Park F, Mattson DL, Roberts LA, Cowley AW., Jr Evidence for the presence of smooth muscle α-actin within pericytes of the renal medulla. Am J Physiol Regul Integr Comp Physiol 273: R1742– R1748, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159– F163, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, Corman B, Verbavatz JM. Downregulation of aquaporin-2 and -3 in aging kidney is independent of V(2) vasopressin receptor. Am J Physiol Renal Physiol 279: F144– F152, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Rocha AS, Kokko JP, Burg MB. Sodium chloride and water transport in the medullary thick ascending limb of Henle: evidence for active chloride transport 1973. J Am Soc Nephrol 10: 1145– 1156, 1999 [PubMed] [Google Scholar]
- 45. Sabolic I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol Cell Physiol 263: C1225– C1233, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Saikia TC. Composition of the renal cortex and medulla of rats during water diuresis and antidiuresis. Q J Exp Physiol Cogn Med Sci 50: 146– 157, 1965 [DOI] [PubMed] [Google Scholar]
- 47. Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA 95: 9660– 9664, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem 266: 6407– 6415, 1991 [PubMed] [Google Scholar]
- 49. Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol Renal Fluid Electrolyte Physiol 271: F414– F422, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Verbalis JG. Escape from antidiuresis: a good story. Kidney Int 60: 1608– 1610, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Verkman AS, van Hoek AN, Ma T, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J. Water transport across mammalian cell membranes. Am J Physiol Cell Physiol 270: C12– C30, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Wang H, Carretero OA, Garvin JL. Nitric oxide produced by THAL nitric oxide synthase inhibits TGF. Hypertension 39: 662– 666, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931– F939, 1996 [DOI] [PubMed] [Google Scholar]