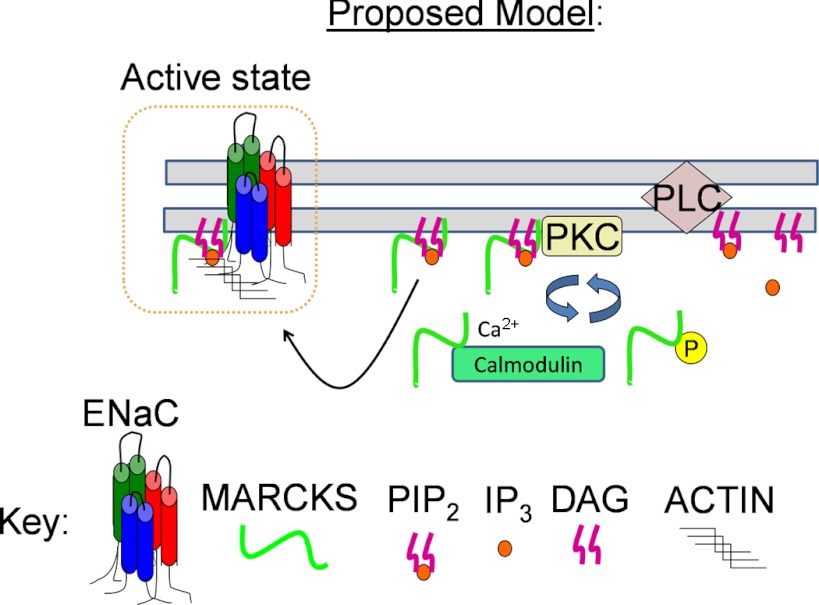
Fig. 13.
Proposed model illustrating that MARCKS acts as a reversible source of PIP2 at the plasma membrane for regulating Xenopus ENaC activity. MARCKS attaches to the cytoplasmic face of the plasma membrane of a quiescent cell, i.e., low free intracellular calcium concentration ([Ca2+]i) and low PKC activity, by its N-terminal myristate and its basic effector domain. MARCKS is displaced from the membrane and resides in the cytoplasm after being phosphorylated by PKC at serine residues in the basic effector domain or upon an increase in free [Ca2+]i, as Ca/CaM binds to the basic effector domain. PIPs bind and activate Xenopus ENaC after MARCKS is displaced from the membrane. PIPs may also stabilize Xenopus ENaC to the actin cytoskeleton and lipid rafts. DAG, diacylglyceride; IP3, inositol-1,4,5-trisphosphate.