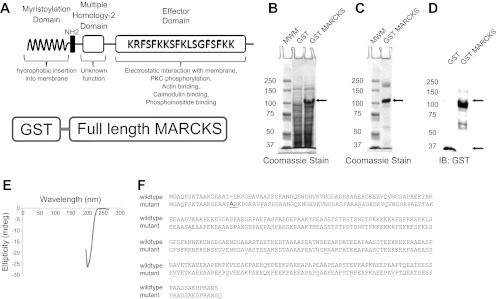
Fig. 4.
Characterization of GST mutant and native myristoylated alanine-rich C-kinase substrate (MARCKS). A: schematic of the GST-MARCKS fusion protein (bottom) and the anatomy of native MARCKS protein (top). The myristoylation domain of MARCKS and the basic effector domain of MARCKS both contribute to the reversible association of MARCKS with the membrane. Binding of the basic effector domain with actin or calcium-calmodulin or posttranslational modification (phosphorylation) of the basic effector domain results in dissociation of MARCKS from the membrane. B: Coomassie-stained gel showing a predominant 100-kDa band after expression of GST-MARCKS with IPTG induction. C: Coomassie-stained gel showing purification of the 100-kDa band after batch purification over Sepharose and eluting with reduced glutathione. D: Western blot analysis of GST-MARCKS confirming a 100-kDa immunoreactive band after probing with a peroxidase-conjugated antibody against GST. E: far UV circular dichroism analysis showing GST-MARCKS is not dominated by secondary structural elements such as α-helixes and β-sheets. F: amino acid sequence alignment comparing native MARCKS with the mutant MARCKS protein.