Abstract
The premature activation of digestive enzyme zymogens in the pancreatic acinar cell is an important initiating event in acute pancreatitis. We have previously demonstrated that vacuolar ATPase (vATPase) activity is required for zymogen activation. Adenosine monophosphate-activated protein kinase (AMPK) regulates vATPase function in kidney and epididymal clear cells. To determine whether AMPK could affect pancreatitis responses, its effects were first examined in a cellular model of pancreatitis, cerulein-hyperstimulated (100 nM) pancreatic acini. This treatment caused a prominent increase in trypsin and chymotrypsin activities. Pretreatment with AICAR or metformin (AMPK activators) or compound C (an AMPK inhibitor) reduced or increased cerulein-induced zymogen activation, respectively. The association of the vATPase E subunit with membranes, a marker of its activation, tended to be inversely related to AMPK activity (assessed by AICAR and compound C treatments). Cerulein treatment did not change AMPK (α and β) levels but did lead to an increase in its activation (phosphorylation of Thr172) and induced the time-dependent translocation of the enzyme to a Triton-insoluble compartment. Basal in vivo studies showed that AMPK was widely distributed between membrane and soluble fractions generated by differential centrifugation. After cerulein hyperstimulation, AMPK levels selectively decreased in fractions containing the highest levels of active zymogens. These studies suggest that AMPK activity has a protective role in the pancreatic acinar cell that inhibits zymogen activation in the basal state, and this AMPK effect is reduced during pancreatitis. Therapies that prevent the selective reduction of AMPK in compartments that support zymogen activation could reduce injury during pancreatitis.
Keywords: pancreatic acini, vacuolar ATPase
the pancreatic acinar cell comprises over 90% of the exocrine pancreas and synthesizes and secretes the enzymes needed to digest nutrients. Many of the digestive enzymes are stored in the acinar cells as inactive zymogens that become activated only after reaching the small intestine. Premature activation of these zymogens within acinar cells appears to have a critical role in initiating acute pancreatitis (17). Experimental models of acute pancreatitis have been developed in which isolated acinar cells or whole animals are treated with supraphysiological concentrations of cerulein (an orthologue of the hormone cholecystokinin) to induce the early stages of the disease.
AMP-activated kinase (AMPK) is a heterotrimeric serine/threonine kinase, composed of α-, β-, and γ-subunits, with the α-subunit possessing the catalytic kinase domain. Phosphorylation of the Thr172 residue in the activating loop of the α-subunit is the primary activator of AMPK (23). Furthermore, AMPK can sense changes in cellular energetics through its two exchangeable nucleotide-binding domains in the γ-subunit; increases in the cellular AMP:ATP ratio enhance the kinase activity through allosteric effects, both promoting phosphorylation and reducing dephosphorylation of Thr172 (6). In addition, increases in ADP concentration also are important in preventing dephosphorylation of the kinase (27).
The pancreatic acinar cell has the highest average rates of protein synthesis in the body (4). This activity consumes high levels of ATP; failure to meet these energy demands can result in protein misfolding and endoplasmic reticulum stress responses. Changes in acinar cell ATP levels have been reported in experimental acute pancreatitis models (20, 25), and the extent of ATP depletion in acute pancreatitis may correspond to disease severity (2). Whether such fluctuations in ATP levels influence AMPK activity in acute pancreatitis is unclear.
In many systems, AMPK regulates the activity of ion transporters. One example is the proton-transporting vacuolar ATPase (vATPase). In the kidney and epididymal clear cells, AMPK activation reduces PKA-mediated accumulation of vATPase in the apical membrane and activation of the transporter (10, 11). vATPase has also been found to have a central role in regulating pancreatitis responses; in the early phases of acute pancreatitis, the vATPase appears to undergo activation (26). Furthermore, chemical or genetic inhibition of the vATPase reduces zymogen activation in the pancreatic acinar cell (15, 26). Together, these findings suggest that AMPK could inhibit acute pancreatitis responses by regulating the acinar cell vATPase.
In this study, we show that modulation of AMPK activity with a chemical agonist or antagonist, respectively, decreases or increases cerulein-induced zymogen activation (as measured by trypsin or chymotrypsin activity), in isolated acini. Cerulein hyperstimulation causes translocation of AMPK into a Triton insoluble compartment. In vivo studies show that this translocation selectively removes it from a subcellular fraction with the greatest levels of activated zymogens. Furthermore, reduction of AMPK levels, or inhibition of active AMPK, is linked with an increase in vATPase translocation and subsequent zymogen activation. Together, these findings suggest that, during pancreatitis, levels of active AMPK may be selectively reduced at the site of zymogen activation in the pancreatic acinar cell. Thus a protective effect of AMPK is diminished early in the course of acute pancreatitis.
MATERIALS AND METHODS
Preparation of isolated pancreatic acini.
Acini were isolated as described (19), with minor modifications. Briefly, fasted male Sprague-Dawley rats 50–150 g (Charles River Laboratories, Wilmington, MA) were euthanized by CO2 using a protocol approved by the Veterans Administration Animal Care and Use Committees. Acinar media was prepared as follows (in mM): 10 HEPES (pH 7.4), 95 NaCl, 4.7 KCl, 0.6 MgCl2, 1 NaH2PO4, 10 glucose, 2 glutamine, plus 0.1% BSA, 1× MEM-amino acids (GIBCO, San Jose, CA), and 1.3 mM CaCl2. The pancreas was collected in 15 ml of calcium-free acinar media. The pancreas was then minced in a minimal volume of calcium-free medium for 5 min and washed three times with the calcium-free medium. The minced tissue was placed into a 50-ml flask with 12 ml of acinar medium containing 50 U/ml of type-4 collagenase (Worthington, Freehold, NJ) for 60 min at 37°C with shaking (120 rpm). The digest was filtered through a 300–400-μm mesh (Sefar American, Depew, NY) and washed with acinar medium. Isolated acini (groups of 20–100 acinar cells) were distributed among the wells (0.5 ml suspension/well) of a 24-well Falcon tissue culture plate (Becton Dickinson, Franklin Lakes, NJ). All reagents were purchased from Sigma (St. Louis, MO), unless otherwise noted.
Acinar cell experimental protocol.
Acini were recovered for 60 min at 37°C under constant O2 with shaking (90 rpm). The media was exchanged for 0.5 ml of fresh media. Pretreatments were added to appropriate wells; these included AICA-riboside (2 mM; EMD Biosciences, La Jolla, CA), compound C (20 μM; EMD Biosciences), and concanamycin (100 nM; Sigma). After 60-min pretreatment, acini were stimulated with cerulein (0.1 nM and 100 nM) for various times. After stimulation, medium and cells were placed in 1.5-ml microcentrifuge tubes (USA Scientific, Waltham, MA) and centrifuged for 1 min at 30 g. A sample (50 μl) of the resulting cell-free supernatant was assayed for amylase content. The remaining 450 μl of cells + media was retained for zymogen activation assays and determination of total amylase. All samples were stored at −80°C.
Enzymatic activity assays.
Enzyme activities were measured as described (5). Briefly, samples were thawed, homogenized, and centrifuged. To each well of a 24-well plate (Falcon 3047) the following was added: 100 μl of postnuclear supernatant, 350 μl of trypsin assay buffer [50 mM Tris (pH 8.1), 150 mM NaCl, 1 mM CaCl2, 0.01% BSA]. The assay was initiated by the addition of 50 μl of 400 μM fluorometric enzyme substrate (trypsin; Peptides International, Louisville, KY; chymotrypsin; Calbiochem division of EMD Chemicals USA, Gibbstown, NJ) diluted in trypsin assay buffer (40 μM final). The plate was read using a fluorometric microtiter plate reader (model HTS 7000; Perkin-Elmer Analytical Instruments, Shelton, CT; 380-nm excitation; 440-nm emission; 20 reads/10 min).
Amylase assay.
Amylase activity was determined by using a commercial kit (Phaebadas kit; Magle Life Sciences, Lund, Sweden) as described (5). Amylase secretion was calculated as the percent total release [medium/ (medium + cells)].
In vivo studies with cerulein.
Male Sprague-Dawley rats (Charles River Laboratories) weighing 150–300 g were fasted overnight and given supraphysiological cerulein (40 μg/kg) via a single intraperitoneal injection (30, 60, or 90 min of stimulation). Animals were euthanized by CO2 using a protocol approved by the Veterans Administration Animal Care and Use Committees.
Subcellular fractionation.
After removal, the pancreas was minced and then gently homogenized in Homogenization Buffer 1 (HB1; 0.3 M sucrose, 10 mM Tris pH 6.4) using a Potter-Elvehjem grinder at low speed. If subcellular fractions were to be used for immunoblots, protease and phosphatase inhibitors were added to HB1: Complete Protease Inhibitor Mini, EDTA-free cocktail (1 tablet per 15 ml stock solution; Roche, Mannheim, Germany), 5 mM benzamidine, 0.1 mg/ml soybean trypsin inhibitor, and PhosSTOP tablet (1 tablet per 10 ml stock solution; Roche). Pancreas homogenate was centrifuged at 600 g for 10 min at 4°C and the pellet resuspended in additional HB1 and recentrifuged. The resulting supernatants were pooled (PNS) and centrifuged at sequential spins (1,800, 3,000, 15,000 g, each 10 min, 4°C and 180,000 and 300,000 g, each 1 h, 4°C) onto a 2 M sucrose cushion. The supernatant from the 300,000 g spin (cytosol), and the pellets from each spin were assayed for enzyme activity and/or protein concentration.
Triton X-100 treatment of 200 g supernatant.
Pancreatic homogenate, prepared as described above, was centrifuged at 200 g for 10 min at 4°C. A final concentration of 1% Triton X-100 was added to 30 μl of the resulting supernatant, gently mixed, and incubated on ice for 10 min. Triton lysates were then centrifuged at 13,000 g for 10 min at 4°C. The resulting supernatant (Triton soluble fraction) was removed and the pellet (Triton insoluble fraction) resuspended in 30 μl 2X Laemmli loading buffer and incubated for 15–30 min at RT before boiling for 5 min at 95°C. Protein content of samples was determined using the Pierce 660-nm protein assay agent with the Ionic Detergent Compatibility Reagent (Pierce Biotechnology, Rockford, IL).
Immunofluorescence.
Rat pancreas was cut into small 2-mm3 cubes, fixed in 4% paraformaldehyde for 2 h at 4°C, incubated in 20% sucrose overnight at 4°C, and frozen in Tissue-tek optimal cutting temperature compound (OCT; Sakura Finetek, Torrance, CA). Alternately, samples were immediately frozen in OCT. Sections, 5 μm thick, were placed on slides and fixed in 2% paraformaldehyde for 2–5 min at room temperature, washed in TBS, and permeabilized in TBS with 0.1% Triton X-100 for 15-min RT. Sections were then quenched with 50 mM ammonium chloride and 5% normal goat serum in TBS for 30 min, rinsed, and incubated with primary antibodies [rabbit anti-AMPK β1/2 (1/50; Cell Signaling Technologies, Danvers, MA) and mouse E-cadherin (1/500; BD Transduction Laboratories, San Jose, CA)] overnight at 4°C. Sections were rinsed twice in TBS with 0.1% Triton X-100, twice in TBS, and incubated with AlexaFluor488 conjugated goat anti-rabbit or AlexaFluor555 conjugated goat anti-mouse secondary antibodies (Molecular Probes, Carlsbad, CA) for 1 h, RT. Sections were rinsed as described above and coverslips mounted in ProlongGold (Invitrogen, Carlsbad, CA) or Vectashield Hard Set Mounting Media with DAPI (Vector Laboratories).
Preparation of cells for immunoblot (vATPase translocation).
Cells were collected after supraphysiological cerulein stimulation for 15 min and centrifuged at 30 g for 1 min. The cell-free medium was removed, and 300 μl of homogenization buffer [25 mM Hepes pH 7.4; 300 mM sucrose; 1 mM benzamidine; Complete Protease Inhibitor Mini cocktail, EDTA-free (1 tablet per 15-ml stock solution; Roche)] was added; acini were then homogenized in a conical 1.5-ml microcentrifuge tube with a pestle and centrifuged at 1,000 g for 5 min. The postnuclear supernatant was removed and recentrifuged at 234,000 g for 15 min at 4°C. The resulting pellet was resuspended in 100 μl of homogenization buffer.
Preparation of cells for immunoblot (AMPK).
Cells were collected, and cell-free medium was reduced to 100 μl. Triton solubilization buffer [200 μl; 20 mM Hepes, pH 7.4, 2 mM EGTA, 20% glycerol, 1 mM DTT, 2% Triton X-100, Complete Protease Inhibitor Mini cocktail (1 tablet per 15-ml buffer, EDTA-free), PhosSTOP tablet (1 tablet per 10-ml stock solution; Roche)] was added, tubes were incubated on ice for at least 10 min, and lysates centrifuged at 13,000 g for 10 min. Alternately, cells were collected, and cell-free medium was reduced to 100 μl. Twenty microliters of 6X Laemmli loading buffer was added, and samples were gently mixed and boiled at 95°C until cell pellets were completely dissolved. Protein content of samples was determined using the Pierce 660-nm protein assay agent with the Ionic Detergent Compatibility Reagent (Pierce).
Immunoblot analysis.
Immunoblot analysis was performed to detect total AMPK, phosphorylated AMPK, protein kinase A, and vATPase subunit V1E. Briefly, samples were separated on SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred to Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked for 60 min at room temperature in Blotto (5% milk in 1X TBS, 0.05% Tween 20; TBS-T). Membranes were washed three times with TBS-T and then probed with primary antibody [AMPK-α, AMPK-β1/2 (57C12), phospho-AMPK-α (Thr172) (40H9), protein kinase A; Cell Signaling Technologies; V1E, Sigma-Aldrich] in 5% BSA in TBS-T overnight at 4°C, washed, and incubated with goat-anti-rabbit or goat-anti-chicken secondary antibody (Sigma-Aldrich) in Blotto for 60 min at room temperature. Membranes were washed in Blotto then TBS-T, and labeled bands were detected using SuperSignal West Pico chemiluminescence (Pierce) or RapidStep ECL reagent (Calbiochem, La Jolla, CA).
Statistical analysis.
Data represent the mean values ± SE of ≥3 individual experiments, with each experiment performed in at least duplicate. Statistical significance was determined by a Student's t-test analysis for in vitro data and the Mann-Whitney test for in vivo data. P < 0.05 designated significance.
RESULTS
AMPK is present in the pancreatic acinar cell.
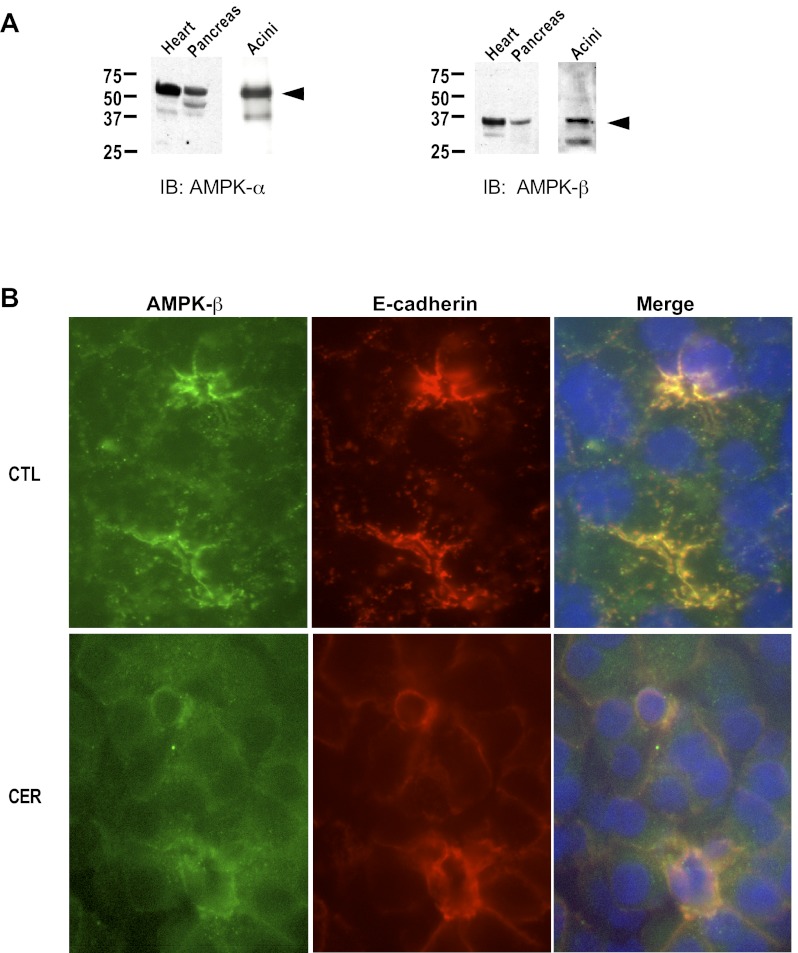
Using antibodies specific to the α- and β-subunits of AMPK, we found that AMPK immunoreactivity is present in both rat pancreas and isolated pancreatic acinar cells (Fig. 1A). This was further confirmed by immunofluorescence; AMPK-β was detected intracellularly on punctate structures throughout the cytoplasm and in the apical region of unstimulated acinar cells, where it colocalized with E-cadherin (Fig. 1B; CTL). Upon cerulein stimulation, AMPK immunoreactivity was dramatically reduced near the plasma membrane. In a subpopulation of cerulein-treated cells, AMPK-β was detected adjacent to the nucleus (Fig. 1B; CER).
Fig. 1.
AMP kinase (AMPK) is present in pancreatic acini. A: representative immunoblot showing presence of AMPK-α (left) and AMPK-β (right) in heart, pancreas, and pancreatic acini (Acini). Arrows indicate relevant band. B: immunofluorescence in rat pancreas: unstimulated (CTL) or treated in vivo with cerulein (CER, 40 μg/kg) for 60 min. Left: AMPK-β (green); middle: E-cadherin (red); right: merge.
AMPK protein levels are reduced in pancreatic acinar cell Triton X-100 lysates after cerulein treatment.
The effects of cerulein stimulation on AMPK activity were investigated by measuring levels of the activating phosphorylation on AMPK-α-Thr172 in isolated groups of pancreatic acinar cells (acini) that were solubilized in Triton X-100. Secretagogue-induced Thr172 phosphorylation over time was assessed by immunoblot and compared with total AMPK-α levels. No significant changes were observed in the levels of pThr172 and total AMPK-α- and -β-subunits when acini were cultured for up to 2 h (Fig. 2, A–C: CTL). However, in the Triton extracts, total pThr172 levels began to decrease 15 min after cerulein stimulation and were significantly reduced by 60 min (Fig. 2A). Surprisingly, total AMPK-α protein levels also significantly decreased over the same period, so no change was observed in the ratio of pThr172 to total AMPK-α protein (Fig. 2B; Table 1). In addition, in Triton extracts, total levels of AMPK-β protein were significantly decreased after cerulein stimulation (Fig. 2C: CER). Protein kinase A (PKA) levels were measured as a control and showed no change with cerulein treatment (Fig. 2D: CTL, CER), suggesting that the reduction in AMPK levels is selective and does not reflect a generalized loss of cytosolic proteins. Because some classes of protein are not solubilized by Triton, we examined the effects of extracting acinar cell proteins with a stronger detergent, SDS.
Fig. 2.
AMPK Thr172 phosphorylation and AMPK levels are reduced in pancreatic acinar cell lysates (Triton X-100) after cerulein treatment. A: phosphorylation of the AMPK activating site (Thr172) decreases when isolated rat pancreatic acini are treated with supraphysiological cerulein (100 nM) over a 90-min time period. Immunoblots show the protein levels of AMPK-α (B) and AMPK-β (C) subunits decrease over time when acinar cells are treated with cerulein, whereas levels of PKA (D) do not. Immunoblots shown are representative of at least 3 similar but separate experiments. Lower graph illustrates densitometry from immunoblots. *P < 0.05 vs. CER time t = 0; n ≥ 3.
Table 1.
Cerulein hyperstimulation does not result in a significant change in the pThr172:AMPK-α ratio in Triton-solubilized cell lysates
| Incubation Time, min | pThr172: AMPK α |
|---|---|
| 0 | 1.000 ± 0.000 |
| 5 | 1.287 ± 0.199 |
| 15 | 1.472 ± 0.478 |
| 30 | 1.298 ± 0.232 |
| 60 | 1.326 ± 0.580 |
| 90 | 1.653 ± 0.518 |
pThr172:AMP kinase (AMPK)-α ratio is calculated at each time point and then expressed as fold vs. time = 0, ± SE, n = 3.
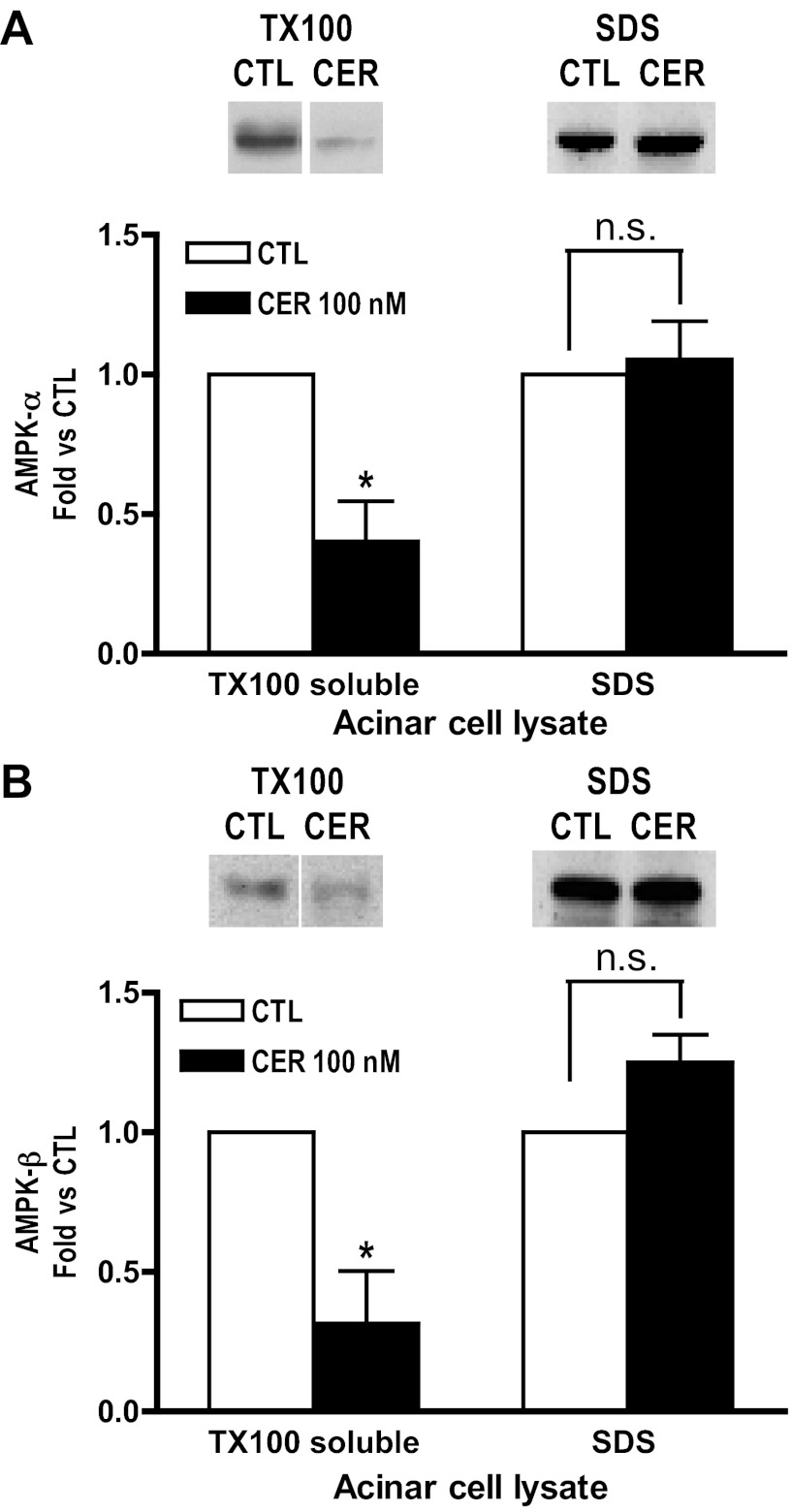
AMPK protein levels are not reduced in pancreatic acinar cell SDS lysates after cerulein treatment.
When acinar cells were lysed with buffer containing SDS (which solubilizes all cellular content), no significant change in the levels of AMPK-α and -β was observed in cerulein-treated cells (60 min) compared with control (Fig. 3, A and B). In contrast, when acini were lysed with Triton under comparable conditions, a reduction in AMPK levels was observed in the Triton-soluble fraction (Fig. 3, A and B). These data suggest that AMPK translocates to Triton-resistant compartment (s) upon cerulein stimulation.
Fig. 3.
AMPK levels following stimulation differ in Triton X-100 (TX100) vs. SDS solubilized acinar cell lysates. Acinar cells were unstimulated (CTL) or treated with cerulein (CER, 100 nM) for 60 min. Samples were solubilized in 1% Triton X-100 or 1.7% SDS (1X Laemmli buffer), and subsequent immunoblots were probed with antibodies specific to AMPK-α (A) or AMPK-β (B). A significant decrease of AMPK levels is observed in TX100 extracts but not in SDS lysates. Top: representative blots. Bottom: densitometry based on 3 to 5 experiments. CTL, open bars; CER, solid bars. *P < 0.05 vs. CTL; n ≥ 3.
AMPK protein content is reduced in the zymogen granule-enriched fractions with in vivo supraphysiological cerulein stimulation.
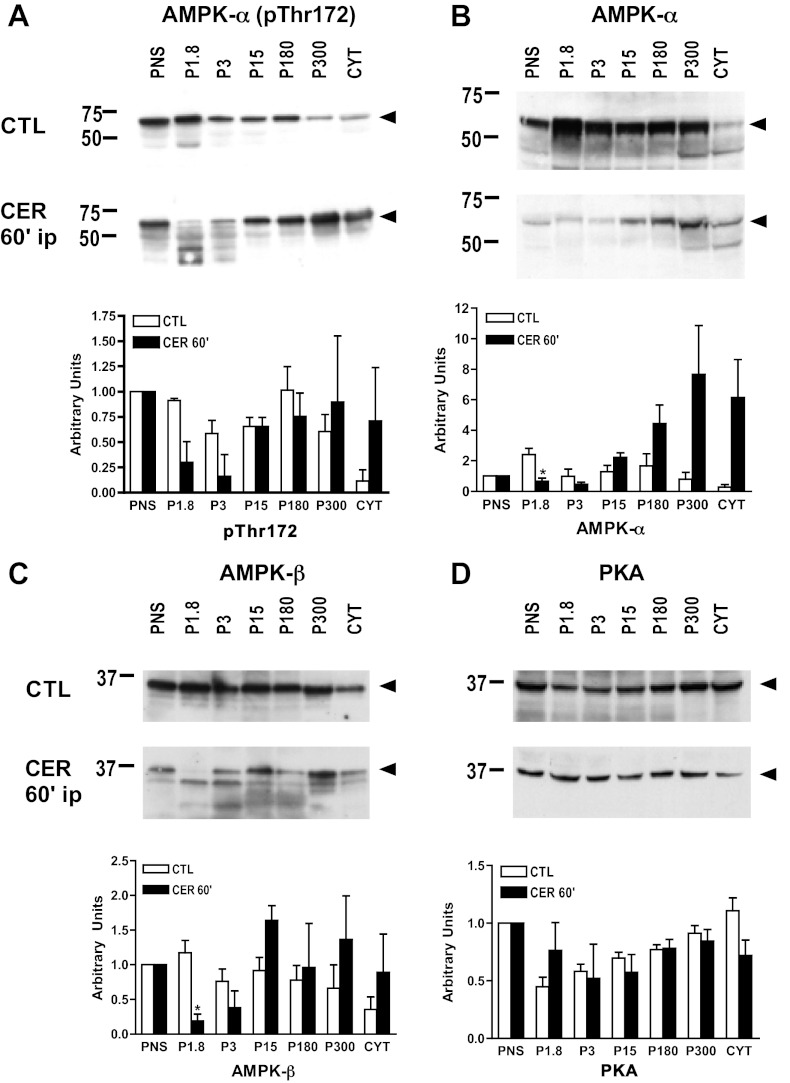
Subcellular fractionation of postnuclear supernatant (PNS; 600-g) using differential centrifugation and immunoblot analysis was performed to determine the distribution of AMPK in rat pancreas. The crude fractions obtained were defined as follows with the predominant organelles found within each pellet also listed: P1.8, 1,800 g pellet (zymogen granules); P3, 3,000 g pellet, (zymogen granules); P15, 15,000 g pellet (lysosomes, mitochondria); P180, 180,000 g pellet (microsomes); P300, 300,000 g (light microsomes); CYT, 300,000 g supernatant (cytosol) (24).
In pancreas from unstimulated animals, the phosphorylated form of AMPK-α (pThr172), total AMPK-α, AMPK-β, and PKA were found in all membrane fractions and cytosol (Fig. 4, A–D: CTL). After inducing pancreatitis in vivo with supraphysiological cerulein, there was a decrease in AMPK subunit levels in the PNS (600 g) (Fig. 5A). Upon further centrifugation of the PNS, AMPK subunit levels were significantly reduced in the zymogen granule-enriched fractions, P1.8 and P3 (1,800 g pellet and 3,000 g pellet, respectively) (Fig. 4, A–C: CER). In contrast, no significant loss of PKA from any of the subcellular fractions was observed (Fig. 4D: CER). Together these findings suggest that following cerulein treatment, AMPK subunit levels selectively decrease in subcellular fractions that are enriched for zymogen granules.
Fig. 4.
AMPK protein expression is downregulated in zymogen-enriched fractions with supraphysiological cerulein stimulation in vivo. Following induction of cerulein pancreatitis in vivo for 60 min, pancreatic fractions were prepared from cerulein (CER)-treated or untreated controls (CTL) rat pancreas using differential centrifugation (see materials and methods). Immunoblot shows the presence of pThr172 (A), AMPK-α (B), AMPK-β subunits (C), and PKA (D) in subcellular pancreatic fractions. Immunoblots shown are representative of 3 similar, but separate, experiments. Lower graph shows quantification of immunoblots using densitometry. *P < 0.05 vs. CTL; n = 3. PNS, postnuclear supernatant; P1.8, 1,800 g pellet; P3, 3,000 g pellet; P15, 15,000 g pellet; P180, 180,000 g pellet; P300, 300,000 g; CYT, 300,000 g supernatant. Arrows indicate relevant band.
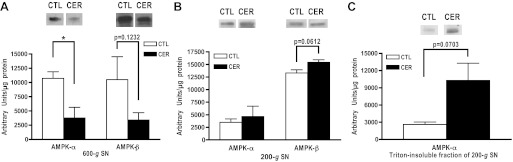
Fig. 5.
With in vivo cerulein hyperstimulation, AMPK subunits translocate to a heavy subcellular compartment that is Triton resistant. Following induction of cerulein pancreatitis in vivo for 60 min, pancreatic homogenates were prepared from cerulein (CER)-treated or untreated controls (CTL) rat pancreas. Homogenate was centrifuged at 200 g or 600 g for 10 min. Immunoblots show presence of AMPK-α and -β in 600 g (A) and 200 g (B) supernatants (SN). Lower graphs show quantification using densitometry. C: 200 g supernatants were treated with Triton X-100 and then centrifuged. The resulting pellet (Triton insoluble fraction) was resuspended in Laemmli sample buffer. Immunoblot shows presence of AMPK-α in Triton-insoluble fraction. Lower graph shows quantification using densitometry. *P < 0.05 vs. CTL; n ≥ 3.
With in vivo cerulein hyperstimulation, AMPK subunits translocate to a heavy subcellular compartment that is Triton-resistant.
Given that AMPK levels decrease in the PNS (600 g) following cerulein hyperstimulation (Fig. 5A; 600 g supernatants), we chose to centrifuge pancreatic homogenates at a lower speed (200 g) to determine whether subunits were moving to a heavier fraction. As shown in Fig. 5B, there was no difference observed in AMPK subunit levels between the 200 g supernatants from unstimulated and cerulein-treated animals. This would suggest that AMPK is translocating to a subcellular compartment that is dense enough to be pelleted at 600 g but not at 200 g.
Data from acinar lysates indicate that AMPK is translocating to a Triton-insoluble fraction (Fig. 3) and in vivo studies suggest that AMPK moves to a heavy compartment (Fig. 5, A and B). To determine whether AMPK present in the heavy compartment is also Triton insoluble, the detergent was added to the 200 g supernatants from unstimulated and cerulein-treated animals and then subjected to a 13,000 g centrifugation. The Triton-insoluble pellets were then solubilized in SDS. With cerulein hyperstimulation, AMPK-α levels increased in the Triton-insoluble fraction compared with control (Fig. 5C). Taken together, the in vivo data suggest that AMPK is translocating to a heavy compartment that is Triton insoluble.
Zymogen activation occurs in zymogen granule-enriched fractions.
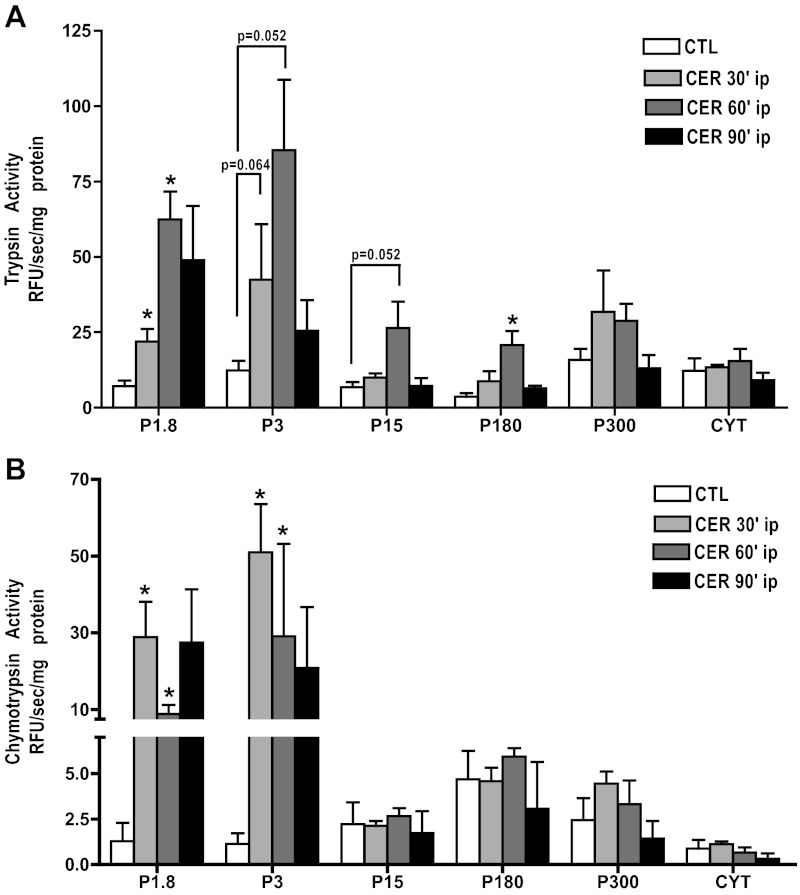
The identity of the subcellular compartment (s) in which zymogen activation occurs during the onset of acute pancreatitis remains unclear. As a first step toward its characterization, we used differential centrifugation to fractionate pancreatic homogenate after in vivo cerulein hyperstimulation. After 30 min of treatment, there were increases in trypsin activity in the zymogen granule-enriched fractions (P1.8, 1,800 g pellet; P3, 3,000 g pellet; Fig. 6A) compared with the control. Similar increases in chymotrypsin activity were also observed (Fig. 6B), but the magnitude of the increase was much greater than that seen with trypsin. Increases in trypsin activity also occurred in the P15 and P180 fractions (15,000 g pellet and 180,000 g pellet, respectively), but these activities were much less than in the heavier fractions. These increases in the lighter fractions were not observed or less pronounced for chymotrypsin activity. After 90 min, trypsin activity had decreased, with only the P1.8 and P3 being higher than control values. These findings indicate that zymogens activated during the initiation of pancreatitis predominately localize to heavy fractions but do not identify the compartment as zymogen granules given the known heterogeneity of the fraction (as assessed by transmission electron microscopy: data not shown).
Fig. 6.
Zymogen activation, in response to cerulein hyperstimulation, occurs in heavy (zymogen granule-enriched) fractions. Following induction of cerulein pancreatitis in vivo for 60 min, pancreatic fractions were prepared from cerulein (CER)-treated or untreated controls (CTL) rat pancreas using differential centrifugation (see materials and methods). Normalized (RFU/s/mg protein) levels of trypsin (A) and chymotrypsin (B) activity in subcellular pancreatic fractions. The highest levels of trypsin and chymotrypsin activity were observed in the heaviest fractions (zymogen granule-enriched, P1.8, P3) from CER-treated animals. *P < 0.05 vs. CTL; n ≥ 3.
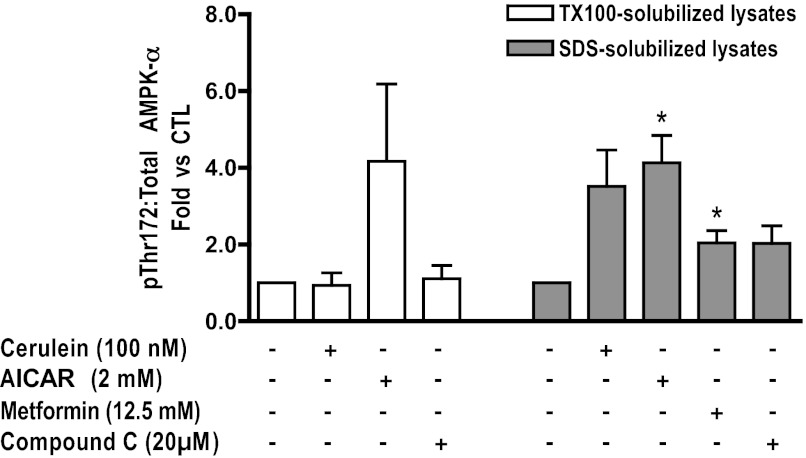
Cerulein hyperstimulation results in an increase in the pThr172:total AMPK ratio in SDS-solubilized lysates.
To determine whether AMPK activity might affect zymogen activation, cerulein-stimulated (60 min) pancreatic acini were pretreated with AMPK activators [AICAR (2 mM) or metformin (12.5 mM)], or an AMPK inhibitor, compound C (20 μM) (7, 9, 28). AMPK activity was estimated by determining the pThr172:total AMPK-α ratio. In unstimulated acini, AICAR and metformin treatment resulted in a significant increase in AMPK activity in SDS-solubilized lysates (Fig. 7; shaded bars). In Triton-solubilized lysates, however, the observed increase in AMPK activity was not significant (Fig. 7; open bars). Compound C did not inhibit basal AMPK activity (Fig. 7). Cerulein treatment caused an increase in AMPK activity in SDS-solubilized lysates (Fig. 7; shaded bars). However, this was not observed in Triton-solubilized lysates (Fig. 7; open bars), suggesting that the Thr172-phosphorylated AMPK selectively partitions into a Triton-insoluble compartment.
Fig. 7.
Effects of AMPK activators and inhibitor on AMPK phosphorylation in pancreatic acini. With cerulein stimulation, AMPK phosphorylation is increased in SDS-solubilized (shaded bars) but not Triton X-100-solubilized (open bars) cell lysates. AICAR and metformin increase the pThr172:total AMPK-α in Triton X-100 and SDS-solubilized cell lysates; compound C does not significantly change the baseline ratio compared with CTL, *P < 0.05 vs. CTL; n ≥ 3.
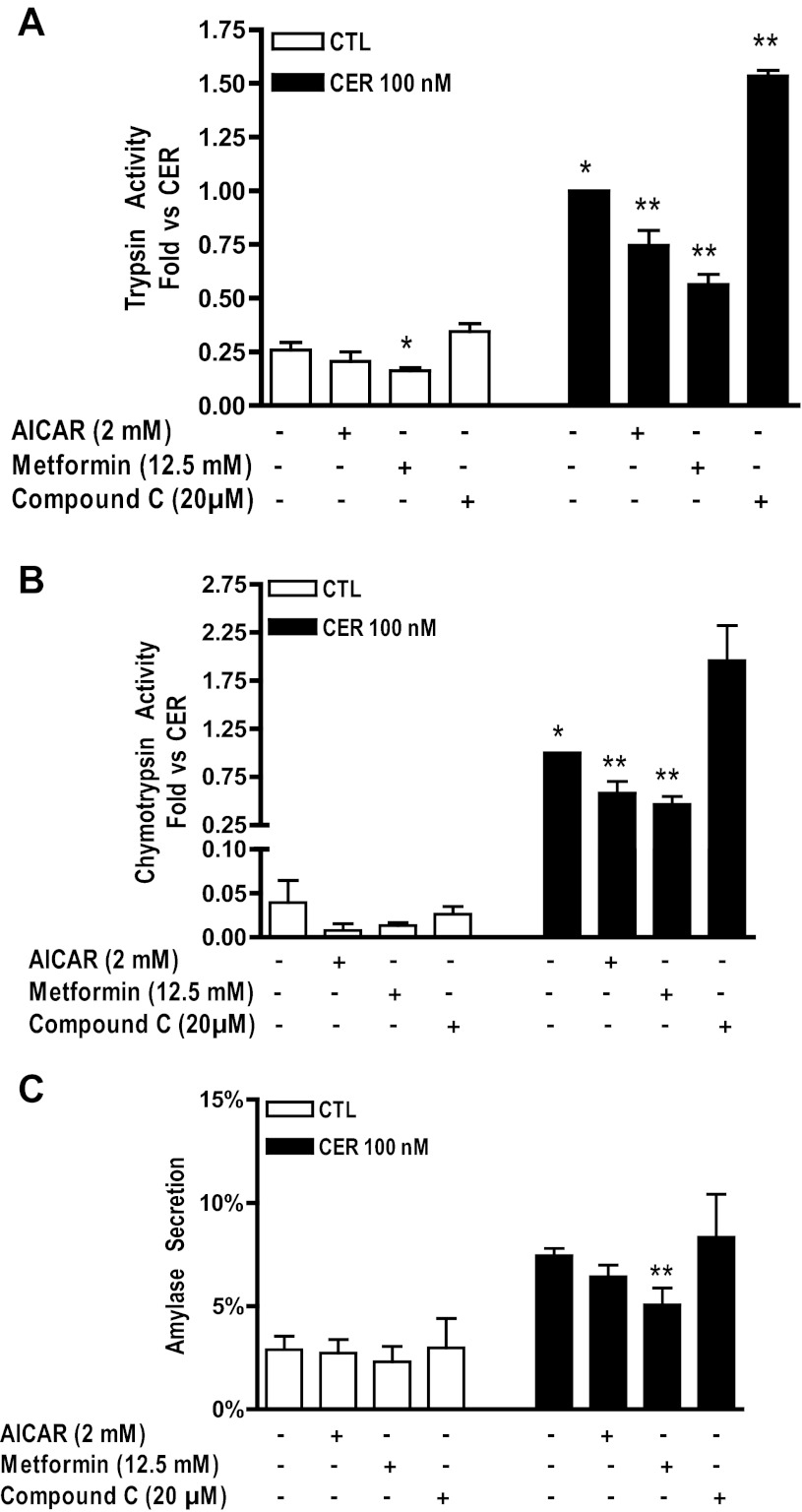
Both AICAR and metformin reduce, and compound C enhances, zymogen activation in isolated acinar cells.
Pretreatment with AICAR or Compound C had no effect; however, metformin pretreatment caused a significant decrease in trypsin activity but did not affect chymotrypsin activity (Fig. 8, A and B). Pretreatment with either AMPK activator (AICAR or metformin) followed by 100 nM cerulein stimulation caused a significant decrease in trypsin and chymotrypsin activity (Fig. 8, A and B). Conversely, pretreatment with the AMPK inhibitor, compound C, significantly enhanced supraphysiological cerulein-stimulated trypsin and chymotrypsin activity (Fig. 8, A and B). Neither AICAR nor compound C affected either unstimulated or cerulein-hyperstimulated amylase secretion at 60 min (Fig. 8C). Treatment with metformin, however, resulted in a significant decrease in cerulein-hyperstimulated amylase secretion.
Fig. 8.
Effects of AMPK activators and inhibitor on zymogen activation in pancreatic acini. Cerulein-stimulated trypsin (A) and chymotrypsin (B) activity are significantly reduced by pretreatment with AICAR (2 mM) or metformin (12.5 mM). Conversely, compound C (20 μM) significantly enhances trypsin activity. C: cerulein-stimulated amylase secretion (CER, solid bars) is significantly reduced by pretreatment with metformin (12.5 mM) but not AICAR (2 mM) and compound C (20 μM). *P < 0.05 vs. CTL; **P < 0.05 vs. CER; n ≥ 3.
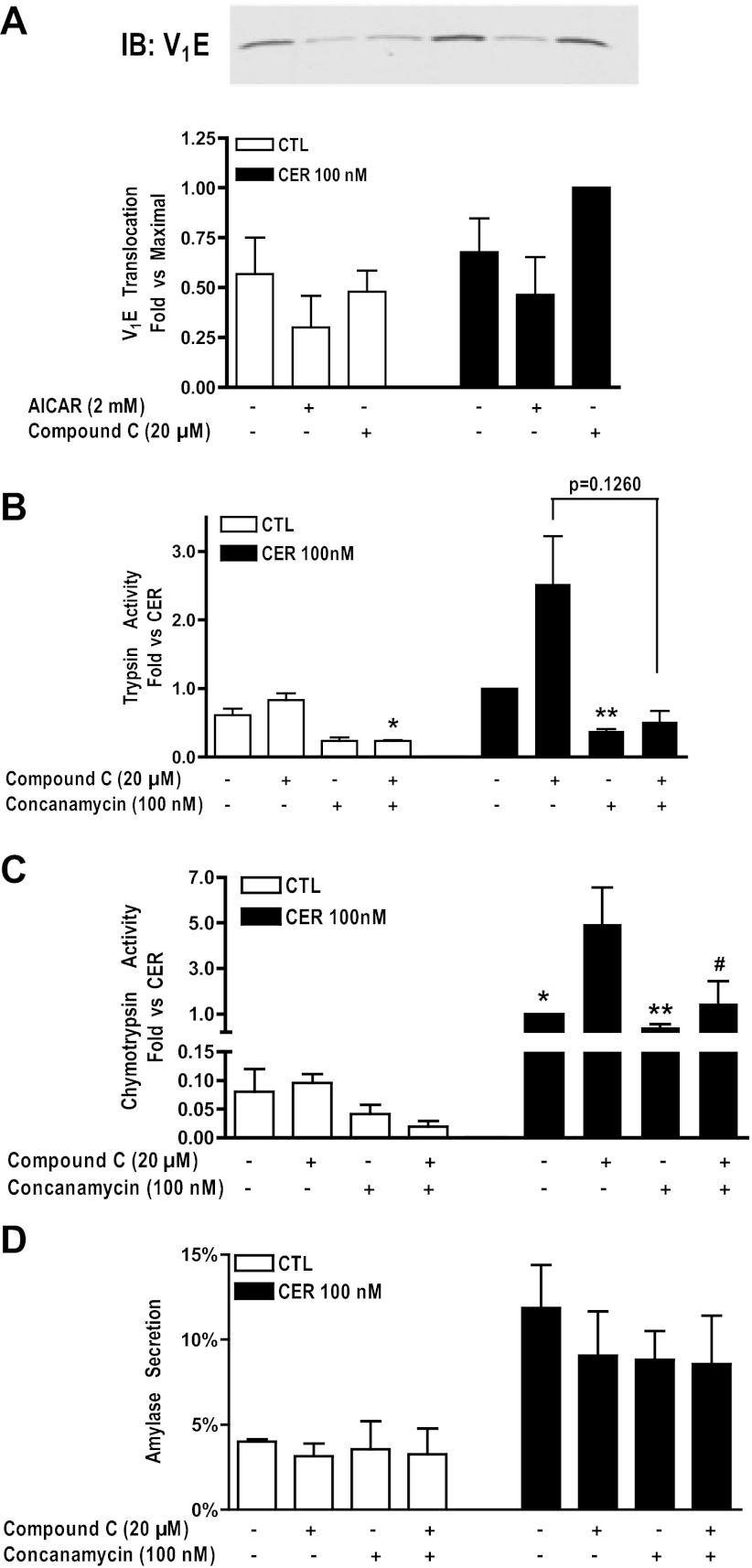
The effect of AMPK is upstream of vATPase activity.
We have shown that the vATPase regulates secretagogue-induced zymogen activation, possibly by regulating acidification of an activation compartment (15, 26). To be a functional transporter, the vATPase must assemble its cytosolic subcomplex (V1) onto its membrane-bound subcomplex (V0). In renal tissues, vATPase translocation to apical membranes is modulated by AMPK (10). vATPase assembly and, indirectly, activity can be assessed by measuring the translocation of V1 subunits to membranes using immunoblot analysis. Acini were pretreated with AICAR (2 mM) or compound C (20 μM) followed by cerulein stimulation for 15 min, an incubation time previously determined to be the optimum for vATPase translocation (13). Pretreatment with AICAR tended to decrease V1 translocation, whereas pretreatment with compound C tended to increase V1 translocation (Fig. 9A) although these results did not reach statistical significance.
Fig. 9.
Effects of AMPK are upstream of vacuolic ATPase (vATPase) activity. A: immunoblot showing the translocation of cytosolic vATPase subunit V1E to a membrane fraction upon CER stimulation of pancreatic acini (CER, solid bars). Immunoblot shown is representative of at least 3 similar but separate experiments. Compound C (20 μM) tended to increase translocation. Cerulein-stimulated trypsin (B) and chymotrypsin (C) activities (CER, solid bars) are enhanced by pretreatment with compound C (20 μM) in pancreatic acini. However, this enhancement is significantly reduced to levels observed in unstimulated cells when acini are pretreated with the vATPase inhibitor, concanamycin. In addition, compound C and concanamycin pretreatment significantly reduced basal trypsin activity. D: cerulein-stimulated amylase secretion (CER, solid bars) is not significantly reduced by pretreatment with compound C (20 μM) and/or concanamycin (100 nM). *P < 0.05 vs. CTL; **P < 0.05 vs. CER; #P < 0.05 vs. CER+compound C; n = 3.
To determine whether AMPK acts upstream of vATPase, acini were pretreated with the vATPase inhibitor concanamycin (100 nM). The inhibitor blocked the enhancement of cerulein-induced zymogen activation induced by compound C (Fig. 9, B and C). The effects seen on trypsin activity trended toward significance, and the effects on chymotrypsin activity were significant. Concanamycin treatment did not significantly change basal or stimulated amylase secretion (Fig. 9D). These data indicate that AMPK acts upstream of vATPase activity, consistent with findings seen in a previous study (10).
DISCUSSION
This study provides an initial characterization of AMPK in pancreatic acinar cells and its potential role in pancreatitis. Immunofluorescence and differential centrifugation studies show that AMPK is associated with multiple acinar cell compartments. Upon induction of pancreatitis with cerulein in acini, AMPK undergoes activation, rapidly dissociates from heavy organelle fractions, and is then sequestered in a Triton-insoluble fraction. Importantly, in vivo pancreatitis studies showed that AMPK is selectively reduced in zymogen granule-enriched fractions that contain the maximal levels of activated zymogens. Furthermore, pharmacological inhibition of AMPK activity resulted in enhanced zymogen activation, suggesting that basal AMPK activation may inhibit this early pancreatitis response. In this context, we found that AMPK activators decreased zymogen activation, indicating that this enzyme might be a therapeutic target for the prevention of pancreatitis.
It is common practice to prepare mammalian cell lysates with a buffer containing detergent (s) (3). In this study, we used both Triton X-100 and SDS for protein solubilization and observed dramatically different results between these detergents. Under basal conditions, AMPK is distributed broadly throughout the acinar cell. With cerulein hyperstimulation, however, AMPK-α and -β protein levels were found to significantly decrease in Triton-solubilized cell lysates but not in SDS-solubilized lysates (Fig. 3, A and B). Translocation of AMPK to a Triton-insoluble compartment after cerulein stimulation could account for these differences.
By assaying the activating phosphorylation of the Thr172 AMPK-α site and normalizing it to total AMPK-α, we found cerulein stimulation resulted in the activation of AMPK in SDS-solubilized lysates (Fig. 7; shaded bars). However, no activation of AMPK was observed in Triton-solubilized lysates after cerulein stimulation (Table 1, Fig. 7; open bars). This suggests that Thr172-phosphorylated AMPK is translocating to a Triton-insoluble compartment.
Pancreatic fractionation studies from in vivo cerulein-hyperstimulated (60 min) rats showed a reduction of AMPK in heavy fractions (P1.8, P3) (Fig. 4, A–D: CER) purified from a 600 g postnuclear supernatant. However, when fractions were prepared from a lower speed (200 g) supernatant, there were no differences in AMPK levels between unstimulated and cerulein-treated animals. This indicates that AMPK is translocating to a subcellular compartment dense enough to be pelleted at 600 g but not at 200 g. Triton treatment of 200 g supernatants further indicates that AMPK partitions to a Triton-resistant compartment after cerulein hyperstimulation (Figs. 5C and 3, A and B).
AMPK localization with, and translocation to, Triton-resistant structures and compartments has been observed in other cellular systems. In endothelial cells, AMPK-α1 has been found to cofractionate with proteins that are selectively expressed in caveolar membranes (8). AMPK-α2 preferentially locates to the nucleus (22), but it also translocates from the cytoplasm to the nucleus in response to exercise or environmental stress (14, 21). On the other hand, AMPK-α1 is predominantly cytoplasmic, but it has been observed to translocate to the nucleus in response to circadian rhythm (16).
A number of cellular compartments, including the nuclear envelope, specialized membrane domains, specific cytoskeletal components, and nuclear proteins can be Triton insoluble. This study does not identify the Triton-insoluble compartment associated with AMPK in the acinar cell although fractionation studies suggest that it is a heavy compartment. Furthermore, the mechanism by which AMPK partitions to a Triton-insoluble compartment is also unknown. Lipid modification of proteins (palmitoylation and myristoylation, in particular) as well as protein phosphorylation promote the association with Triton-insoluble fractions. For example, connexin 43 translocates to Triton-insoluble membrane domains after it is phosphorylated by PKC-γ (13, 18). Goals of our future studies will include identifying the Triton-insoluble compartment in which AMPK is sequestered and defining the signaling pathways that imparts this property.
The cerulein-stimulated loss (15–60 min) of active AMPK from the site of zymogen activation to a Triton-insoluble compartment is a major finding of this study and likely contributes to the rapid activation of zymogens at the onset of acute pancreatitis. Thus AMPK activity in the basal state may inhibit zymogen activation, and AMPK inactivation could promote this zymogen activation. The effects we observed using pharmacological manipulation of AMPK in a cellular model of pancreatitis (cerulein-stimulated acini) support this concept. The presence of basal AMPK activity, or its chemical activation with AICAR or metformin, reduces zymogen activation in cerulein-stimulated acini (Fig. 8, A and B). In contrast, inhibition of AMPK activity with compound C enhances zymogen activation (Fig. 8, A and B). The inhibitory effects of AMPK on zymogen activation may be linked to its inhibition of the proton transporter, the vacuolar ATPase, a key mediator of zymogen activation (Fig. 9A).
Although the effects of pharmacological agents must be cautiously interpreted, the two AMPK activators used in this study, AICAR and metformin, have distinct mechanisms of action but had the same effect on zymogen activation (Fig. 8, A and B) (7, 12, 28). Compound C is a potent AMPK inhibitor but also has a broad pharmacological profile that could have contributed some of the effects we observed in this study (1). Nonetheless, the collective effects of these two AMPK agonists and AMPK inhibitor strongly suggest that AMPK activity is inversely related to zymogen activation.
To determine whether the effects of AMPK are upstream of vATPase, a mediator of zymogen activation, preparations of acini were treated with the vATPase inhibitor, concanamycin (Fig. 9, B and C). The enhancement of zymogen activation seen in the presence of compound C was blocked by concanamycin, suggesting that AMPK effects are upstream of vATPase activity. Modulation of AMPK appeared to affect assembly of vATPase, a key requirement for its activation. This observation is consistent with other studies showing an inverse relationship between AMPK activity and translocation-dependent vATPase activation (Fig. 9A) (17). Together, these findings suggest that, in the pancreatic acinar cell, the effect of AMPK on protease activation may be due, in part, to inhibition of vATPase assembly.
In summary, this study characterizes the distribution and function of AMPK in the pancreatic acinar cell during acute pancreatitis. AMPK is associated with heavy subcellular fractions containing zymogen granules and other organelles. Under basal conditions, AMPK activity inhibits zymogen activation, whereas AMPK inhibition is associated with enhanced zymogen activation. Cerulein hyperstimulation causes the rapid phosphorylation of AMPK on Thr-172, partitioning of the enzyme to a Triton-insoluble compartment, and its selective loss from fractions that contain the most activated zymogens. The effects of AMPK on zymogen activation are likely mediated by its effects on the assembly and/or function of the vATPase, a pivotal mediator of zymogen activation. The dissociation of AMPK from organelles that are enriched in digestive enzymes may eliminate the AMPK suppression of vATPase activity and promote the vATPase-dependent zymogen activation. These findings may have clinical implications for the use of pharmacological activators of AMPK, such as the diabetes medication metformin, as an attractive prophylactic therapeutic option reducing injury in acute pancreatitis.
GRANTS
This work was supported by NIH RO1 DK54021 and VA Merit Award to F. Gorelick and NIH RO1 HL63811 to L. Young and NIH R21 AA020847-01 to E. C. Thrower.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.S., M.A., A.P.D.D.V., T.R.K., L.H.Y., F.S.G., and E.C.T. conception and design of research; C.A.S., M.A., A.P.D.D.V., and T.R.K. performed experiments; C.A.S., M.A., A.P.D.D.V., and T.R.K. analyzed data; C.A.S., M.A., A.P.D.D.V., T.R.K., L.H.Y., F.S.G., and E.C.T. interpreted results of experiments; C.A.S., M.A., and A.P.D.D.V. prepared figures; C.A.S., M.A., A.P.D.D.V., T.R.K., and E.C.T. drafted manuscript; C.A.S., T.R.K., L.H.Y., F.S.G., and E.C.T. edited and revised manuscript; C.A.S., L.H.Y., F.S.G., and E.C.T. approved final version of manuscript.
REFERENCES
- 1. Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binker MG, Binker-Cosen AA, Richards D, Gaisano HY, de Cosen RH, Cosen-Binker LI. Chronic stress sensitizes rats to pancreatitis induced by cerulein: role of TNF-alpha. World J Gastroenterol 16: 5565–5581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonifacino JS, Dell'Angelica EC. Immunoprecipitation. Curr Protoc Cell Biol Chapter 7: 72, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc 53: 211–354, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Chaudhuri A, Kolodecik TR, Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 288: G235–G243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature 459: 1146–1149, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-Carboxamide Ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells. Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Creighton J, Jian M, Sayner S, Alexeyev M, Insel PA. Adenosine monophosphate-activated kinase α1 promotes endothelial barrier repair. FASEB J 25: 3356–3365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Sheehan M, Hirst W, Michel AD, Randall A, Hardie DG, Frenguelli BG. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem 88: 1272–1282, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420–2425, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, Kass DA, Machamer CE, Van Eyk JE, Tomaselli GF. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res 106: 1153–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK–>ERK1/2 pathway. Am J Physiol Cell Physiol 293: C1427–C1436, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kolodecik T, Gorelick F, Thrower E. Genetic and pharmacologic manipulation of vacuolar atpase; effects on zymogen activation in pancreatic acini. Open Access Anim Physiol 2009: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest 87: 362–366, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin D, Lobell S, Jewell A, Takemoto DJ. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase Cg. Mol Vis 10: 688–695, 2004 [PubMed] [Google Scholar]
- 19. Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 282: G501–G507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luthen R, Niederau C, Grendell JH. Intrapancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 268: G592–G604, 1995 [DOI] [PubMed] [Google Scholar]
- 21. McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M. Exercise increases nuclear AMPK alpha2 in human skeletal muscle. Diabetes 52: 926–928, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J 334: 177–187, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437–443, 2000 [PMC free article] [PubMed] [Google Scholar]
- 24. Tartakoff AM, Jamieson JD. Subcellular fractionation of the pancreas. Methods Enzymol 31: 41–59, 1974 [DOI] [PubMed] [Google Scholar]
- 25. Voronina SG, Barrow SL, Simpson AW, Gerasimenko OV, da Silva Xavier G, Rutter GA, Petersen OH, Tepikin AV. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology 138: 1976–1987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem 280: 5430–5434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]