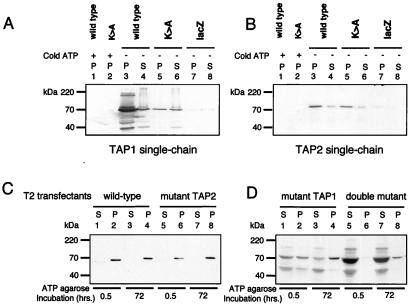
Figure 2.
Mutant TAP1 and TAP2 polypeptides retain the ability to bind ATP. (A and B) Single-chain TAP polypeptides can bind to ATP-agarose. Subconfluent HeLa cells were infected with vTF7-3 and then transfected with vectors encoding wild-type and mutant TAP polypeptides and a control lacZ (as indicated). After overnight incubation, the cells were extracted in 1% Triton X-100 and incubated with 50 μl of glycerol for 4 days and ATP-agarose for a further 24 h. Equal cell equivalents of the ATP-agarose bound pellet (P) and unbound supernatant (S) fractions were separated on a 10% SDS/PAGE gel, transferred onto poly(vinylidene difluoride) (PVDF) membranes, and probed with (A) TAP1 (148.3) mAb and (B) TAP2 (435.3) mAb. (C and D) Wild-type and mutant TAP-transfected T2 bind ATP-agarose. T2 transfectants were extracted in 1% Triton X-100, and, after incubation with ATP-agarose for 0.5 and 72 h, the bound (P) and unbound (S) proteins were separated by 10% SDS/PAGE and probed with the TAP1 (148.3) mAb. Reactive bands were detected by chemiluminescence; the exposure in D was 10 times longer than in C.