Abstract
In order to better understand and treat neuropathic pain, scientific study must use methods that can assess pain processing at the cortical level where pain is truly perceived. Operant behavior paradigms can accomplish this. We used an operant task to evaluate changes following chronic constriction injury to the trigeminal nerves. We also relate these behavioral changes to immunohistochemistry of transient receptor potential channels vanilloid 1 and melastatin 8 (TRPV1 and TRPM8) in the trigeminal ganglia. Following nerve injury, successful performance of the operant task was reduced and aversive behaviors were observed with 10 and 37°C stimulation, indicating cold allodynia and mechanical allodynia respectively. In contrast, while aversive behaviors were observed with 48°C stimulation, successful performance of the operant task was not substantially hindered following injury. These behavioral changes were accompanied by an increase in TRPV1 positive cells and an increased intensity of TRPM8 staining at two weeks post-injury, when cold allodynia is maximal. These findings suggest that the incorporation of operant behavioral assessment in the study of pain may provide insight into the relationship among peripheral changes, motivational drive, and pain. Understanding this relationship will allow us to better treat and prevent chronic neuropathic pain.
Keywords: trigeminal neuropathic pain, operant, TRPM8, cortical processing, allodynia, TRPV1
Chronic pain in the orofacial region is experienced by 25% of the United States, including disorders such as trigeminal neuralgia and pain associated with trauma to the face and mouth (Lipton et al., 1993). However, the mechanisms underlying these conditions are not completely understood, particularly with respect to neuropathic pain. To fully understand trigeminal neuropathic pain it is important to utilize models and behavioral assessment that directly impact and measure the trigeminal system, as differences have been noted between neuropathic injury models affecting extra-cephalic and cephalic sites (Benoliel et al., 2001, Latrémolière et al., 2008, Kayserl et al., 2011). Furthermore, it has become increasingly evident that chronic, maladaptive pain is maintained by changes at the cortical level (de Leeuw et al., 2005, Seifert and Maihofner, 2009). Therefore, the evaluation of trigeminal pain and analgesia in experimental animals should incorporate methods that directly evaluate cortical processing by requiring that the animal make a decision about its environment.
Operant assays directly evaluate cortical processing of nociceptive input (Vierck et al., 2008). Additionally, operant tests more closely resemble functional assessments that are made clinically. That is, operant tests “ask” the animal subject if the pain they are experiencing hinders them from performing functionally important or rewarding tasks. More groups are beginning to use operant methods to evaluate thermal allodynia and hyperalgesia following sciatic or spinal nerve injury (Vierck et al., 2005, Jabakhanji et al., 2006, Walczak and Beaulieu, 2006). However, operant assessment of thermal hyperalgesia following trigeminal nerve injury is currently limited to one publication (Kumada et al., 2012).
In this study, we sought initially to determine the effects of bilateral chronic constriction injury (CCI) of the infraorbital trigeminal nerves on thermal sensitivity in the face, using an operant assay that we have previously characterized (Neubert et al., 2005, Neubert et al., 2006, Rossi et al., 2006). We assessed responses to cold, hot, and neutral stimulation before and up to four weeks after surgery. The thermode set at the neutral (body) temperature may be a surrogate for mechanical sensitivity. In addition to operant assessment, we also used a behavioral scoring system concurrent with operant testing to assess changes in aversive behaviors exhibited with stimulation. These behavioral assessments were also accompanied by assessment of nerve inflammation and injury and immunohistochemistry against the transient receptor potential (TRP) channels vanilloid 1 and melastatin 8 (TRPV1 and TRPM8, respectively) in trigeminal ganglia from sham- and CCI-treated rats. Changes in TRP channel expression, particularly TRPV1 (Christoph et al., 2006), have been implicated in peripheral sensitization that contributes to neuropathic pain, and evidence also suggests that TRPM8 may play a role as well (Xing et al., 2006, Xing et al., 2007, Su et al., 2012). The incorporation of operant behavioral assessments may allow us to better understand the relationship between processes involved in establishing neuropathic pain states and changes in motivation and behavior that impact pain. Understanding this relationship is critical for improving treatment and developing ways to prevent neuropathic pain.
Experimental Procedures
Animals
Male hairless Sprague-Dawley rats (n = 45, five to seven weeks old, Charles River, Raleigh, NC) were housed in groups of four in enriched housing (see (Rossi and Neubert, 2008) for description of enrichment). All rats were maintained in a standard 12-hour light/dark cycle and were allowed access to food and water ad libitum when not being tested. Rats’ weights were recorded every week to monitor general health. Animal testing procedures and general handling complied with the ethical guidelines and standards established by the Institutional Animal Care & Use Committee at the University of Florida (Institute of Laboratory Animal Resources, 1996).
Induction of Neuropathic Pain
Following training and baseline behavioral testing, rats received either a bilateral chronic constriction injury (CCI) or sham operation of the infraorbital portion of the trigeminal nerve (maxillary division). The operant chambers are designed to stimulate both sides of the face simultaneously, so an ipsilateral versus contralateral comparison would be difficult to make. This is why we opted to perform a bilateral injury. These surgeries were performed intraorally, as described by Imamura and colleagues (Imamura et al., 1997), with some modification. We chose this intraoral route rather than an external method so that the incision site would not be within the field of thermal testing. Briefly, rats were deeply anesthetized with a ketamine/xylazine cocktail (2:1, 1.2mg/kg). For local anesthesia and hemostasis, xylocaine (2%, 1:100,000 epinephrine) was applied to the intraoral incision area. An incision was made beginning from the hard palate and extending approximately 1 cm anteriorly toward the maxillary incisors, roughly parallel with the lip. The nerve was exposed from surrounding tissues by blunt dissection and gently elevated with a hooked instrument so that two ligatures (5-0 vicryl suture) could be tied securely around the nerve. This procedure was the same for sham surgeries, except that sutures were passed under the nerve, but not tied. This process was repeated for the second side. All incisions were closed with 2–4 ligatures (5-0 vicryl suture). All surgeries were performed by the same investigator and nerves were treated in the same order each time. Rats’ weights, general behavior, and facial scratching were tracked for one week after the surgeries to monitor healing and recovery. A blinded observer noted the location and extent of scratching within the innervated area. Behavioral testing began after the one-week recovery period. Three sets of surgeries were conducted, for a total of 18–22 rats assigned to each treatment.
Behavioral Testing
Operant Evaluation of Thermal Sensitivity
Facial testing was conducted using a reward-conflict operant testing paradigm as described previously (Neubert et al., 2005). In this paradigm, the rat may choose to experience thermal stimulation that may be painful in order to obtain a milk reward, or they may abstain from both reward and experiencing pain. Briefly, rats were trained to drink sweetened condensed milk while making facial contact with a single thermode and lick-tube. During the training period (approximately 2 weeks) baseline intake was recorded, and rats were considered ready for experimental testing once their average baseline intake was 10 g or greater at 37 °C. The facial testing region for each animal was depilitated under light isoflurane anesthesia (inhalation, 2.5 %) once a week to maximize thermal stimulus contact. Rats were fasted for 13–15 hrs prior to stimulus testing, except for 37 °C.
Following training, baseline data was recorded twice for each stimulus temperature (10, 37, and 48 °C). For every 20-minute testing session, three outcome measures were calculated for each rat as described previously (Neubert et al., 2005): number of licks, number of stimulus contacts, and the pain index, which is the number of licks (successful attempts) divided by the number of stimulus contacts (total attempts). Following surgical treatment and recovery, rats were tested with one of the following schedules: three times at 10 °C and once at 37 °C per week; twice at 10 °C, once at 37 °C, and once at 48 °C per week; or once each at 10, 37, and 48 °C per week. We were initially interested in characterizing cold allodynia, as this is of more clinical relevance than heat hyperalgesia. In subsequent groups of rats we included a hot stimulus to determine if heat hyperalgesia was also present. It should be noted that rats were fasted prior to each stimulus, except for 37°C, with no more than three overnight fasts per week.
Evaluation of Aversive Behaviors during Operant Testing
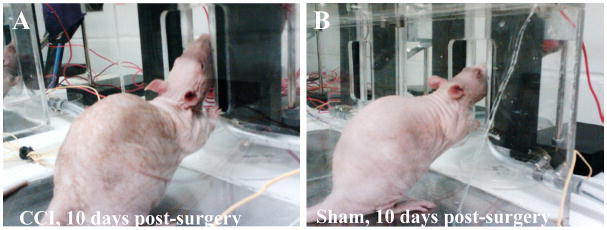
During the first cycle of testing it was noted that some rats would tilt their heads in an effort to displace the stimulus away from the portion of the face innervated by the infraorbital nerve (Fig. 1, and supplemental video). The experimenter who made this observation was blinded to treatment. The rats exhibiting this behavior were later identified as CCI-treated animals. Thus for subsequent groups of surgically treated animals, a system of scoring was established to assess additional innate and aversive behaviors directed towards the stimulus in addition to changes in operant outcomes. Rats were observed during the first five minutes of operant testing and one point was assigned for the presence of three aversive behaviors (head tilting, wiping or pushing at the stimulus with forepaws, and biting at the stimulus). We chose to assess the first five minutes of testing rather than the entire testing period because untreated rats attend to operant task completion immediately and typically do not engage in grooming or other behaviors unrelated to the operant task until they completed at least one bout of drinking. Five naïve rats were tested in parallel with one group of surgically treated rats to determine if aversive behaviors might occur spontaneously at any of the stimuli tested. This and all behavioral testing described above was assessed by a treatment-blinded investigator.
Figure 1.
CCI-treated rats exhibit aversive behaviors towards the stimulus not observed in Sham-treated rats. A) A CCI-treated rat tilting his head upward, shifting contact away from the mystacial pad affected by the constriction injury. B) A sham-treated rat, which underwent surgery on the same day as the rat on the left and was also tested within the same session. Naïve animals have the same posture as the sham-treated animals (not shown).
Histology
Tissue Preparation
Rats were euthanized by isoflurane asphyxiation and decapitation at several postoperative time points (2, 5, 6, 7, and 12 weeks). The infraorbital trigeminal nerves and trigeminal ganglia were dissected free and post-fixed in 10% neutral buffered formalin for 48 hours before being transferred to 70% ethanol. Tissues were embedded in paraffin blocks and 10 μm sections were cut with a microtome and mounted on slides. At least two non-adjacent sections, separated by 50 μm, were taken for each pair of nerves and each pair of ganglia. Tissues were de-parafinized and rehydrated in a graded series of alcohols into PBS. Immunohistochemistry was performed on sections of trigeminal ganglia to visualization TRPV1 and TRPM8 proteins and quantify the number of immunoreactive cells.
Assessment of Nerve Inflammation and Injury
Assessment of nerve inflammation and injury was made by a blinded investigator. Two longitudinal, non-adjacent sections of the left and right nerves from each individual were assessed in the approximate area of ligation or sham ligation. These four sections were evaluated for presence and degree of nerve damage, as well as infiltration of immune cells. The microscopic presence of suture material and foreign body reaction was noted for each of the four sections. If microscopic suture material was noted in any of the four sections viewed per animal, then that animal was counted as having suture.
Immunohistochemistry
Target retrieval was used for immunostaining of TRPV1 only. TG sections were incubated in 100 ml target retrieval solution (Dako Cytomation North America, Carpinteria, CA) for 15 min in boiling water, then incubated in an oven overnight at 60°C overnight. On the second day, sections were cooled and washed with 0.1% Tween20 in PBS. They were then blocked with 10% normal goat serum (NGS) for 30 min at room temperature, and incubated with rabbit anti-TRPV1 antibody (1:500, Affinity Bioreagents; Golden, CO) in 5% NGS in 0.1% Tween20 in phosphate buffered saline (PBS, the antibody diluent) overnight at 4 °C. This process was the same for immunostaining of TRPM8 with the following changes: target retrieval was not performed and the chicken anti-TRPM8 antibody (1:500, Aves, Tigard, OR) was incubated for two days at 4 °C.
After incubation in the primary antibody, sections were washed and incubated with the appropriate secondary antibody in the antibody diluent at 1:1,000 (for TRPV1; goat anti-rabbit IgG AlexaFluor 488 and for TRPM8; goat anti-chicken IgY Alexa Fluor; Invitrogen). Slides were then washed in PBS and coverslips applied with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA). Immunostained sections of trigeminal ganglia were viewed with a Micro-Leica DMLBZ microscope at 200 times magnification, and images were captured using the Q Imaging Micropublisher 5.0 camera and Q Capture Pro software. The area of the trigeminal ganglion innervated by the infraorbital nerve was surveyed and cell counts were made using three representative images from each ganglion from each individual (n = 6 views from 3–4 rats per treatment). For intensity of TRPM8 immunoreactivity, all TRPM8 positive cells in two images per rat from each treatment group were analyzed using ImageJ 1.4g (National Institutes of Health).
Statistical Analysis
For analysis of behavioral data following surgical treatment, outliers were first removed from all data sets using a box plot analysis (SPSS, v.16), on an outcome by outcome basis. Outliers are defined as any value beyond one and a half times the interquartile range. General linear models for two-way Analyses of Variance (ANOVAs) were used to assess the effects of surgical treatment, time and their interaction on operant outcome measures and aversive behavior scores for all stimuli tested, as well as the percentage of TRPV1 expression, and TRPM8 intensities. One way-ANOVAs were used to assess the effect of time within each surgical group, followed by post-hoc Tukey’s test. Unpaired t-tests were used to make comparisons between behavioral outcome measures of CCI- and sham-treated rats at each post-operative time point. Statistical significance was set at p<0.05 for all analyses.
Results
General Observation of Immediate Post-Surgical Recovery and Behavior
Rats’ weights were recorded before surgery and monitored daily after surgery to track recovery (Table 1). CCI-treated rats lost an average of 3% of their body weight at 24hrs post-op, but recovered to 99% of their preoperative weight by the seventh day. In contrast, sham-treated rats maintained their pre-operative weight at 24hrs post-surgery, and gained an additional 5% on top of their preoperative weight by the seventh day. While there was a significant effect of surgical treatment on weight (two-way ANOVA, F= 10.77, p = 0.0011), there were no post-hoc differences between CCI- and Sham-treated rats on any post-operative day. Additionally, the post-operative weights for both groups were not significantly different from their pre-operative weights. Thus, the CCI-treatment was associated with a delay in normal weight gain, but not substantial weight loss. This indicates that the impact of the treatment on normal feeding was minor.
Table 1.
Mean weights of rats pre- and post-surgery (±SEM, n = 19–20/treatment*)
| Days Post-Surgery | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Sham | 350 ± 5 | 352 ± 5 | 353 ± 6 | 358 ± 6 | 362 ± 6 | 361 ± 6 | 366 ± 9 | 375 ± 5 |
| CCI | 354 ± 6 | 345 ± 6 | 344 ± 6 | 348 ± 6 | 349 ± 7 | 344 ± 7 | 345 ± 9 | 349 ± 13 |
n = 13–14/treatment for day 6 and n = 5–7/treatment for day 7.
All surgically treated rats were observed engaging in isolated facial grooming on recovering from anesthesia. In particular, some CCI-treated rats would swat at the rostral portion of their faces with their forepaws, in a motion reminiscent of normal face washing, but without contact to the face and often interspersed with forepaw shaking. Some CCI-treated rats were also observed jerking or jumping as if in response to little or no apparent facial contact with their environment. These behaviors might be indicative of severe spontaneous pain, but jerking and jumping was not observed consistently after the first two postoperative days. Scratching was noted in the surgical area of fifteen CCI-operated rats, and in nine sham-operated rats (n = 19–20/treatment). This behavior was more severe and longer lasting in the CCI-operated rats, but resolved and healed by the end of one week.
Occurrence of Avoidance Displays in Operant Task Following Chronic Constriction Injury
With the first group of surgically treated rats, it became apparent that many of the CCI-treated rats were exhibiting nocifensive behaviors towards the stimulus. In particular, many CCI-operated rats adopted an upward head-tilt, not observed in their sham-operated counterparts during this time period (Fig. 1). This response likely indicates mechanical allodynia. Rats exhibiting this strategy would initially place their faces with the dermatome innervated by infraorbital nerve against the thermode as they had been trained. As contact with the 10 °C thermode continued, the rat would tilt his head upward, shifting contact to a more caudle portion of the face not innervated by the constricted portion of the nerve. Early in testing some individuals exhibiting this head tilt would jerk back on first contact. This head tilt was also accompanied by paw wiping, pushing, or in some cases biting at the thermode. These behaviors were first identified by a treatment-blinded investigator. We have never previously observed this head tilting in naïve rats or following any other type of treatment using this assay. This includes carrageenan-induced inflammation (Neubert et al., 2005), menthol (Rossi et al., 2006), capsaicin (Neubert et al., 2007), and substance P conjugated cholera toxin in combination with naloxone (Caudle et al., 2010).
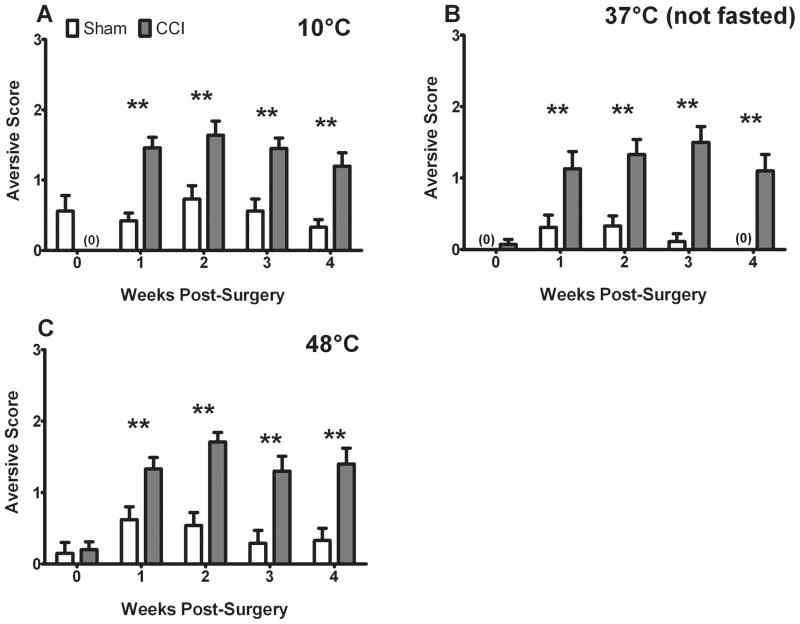
To systematically assess these avoidance displays, a scoring system was used to determine the frequency of aversive behaviors directed at the 10, 37, or 48 °C thermodes in the first five minutes of operant testing. In order to reconfirm that aversive displays are not exhibited consistently in the absence of pain, five naïve rats were scored in tandem with a subset of surgically treated rats across 25 to 35 testing sessions. A blinded observer indicated that naive rats never exhibited head tilting. They did display the other two aversive behaviors occasionally, producing an average score of 0.3 ± 0.1 out of 3 with 10°C stimulation and 0.04 ± 0.04 with 48°C stimulation (mean ± SEM).
In contrast, aversive behaviors were consistently observed following CCI-treatment (Fig. 2). We observed a significant effect of treatment (10°C: F = 33.907, 37°C: F = 62.409, 48°C: F = 11.67, all p < 0.001), a significant effect of time (10°C: F = 7.791 p < 0.001, 37°C: F =8.076 p < 0.05, 48°C: F = 10.913 p < 0.001), and a significant interaction (10°C: F = 7.791 p < 0.001, 37°C: F = 4.535 p < 0.05, 48°C: F = 4.021 p < 0.05) on aversive behaviors for all three stimuli tested.
Figure 2.
CCI is associated with increased aversive behavior scores in the presence of thermal stimulation. Aversive behaviors were persistently elevated for 10°C (A), 37°C (B), and 48°C (C) following CCI treatment, as compared to both baseline and their sham counterparts (indicated by ** ANOVA and t-tests). P< 0.05 and all data are mean ± SEM.
Effect of Surgical Treatment on Reward Licks, Facial Contacts with the Stimulus, and Pain Index (licks/contacts)
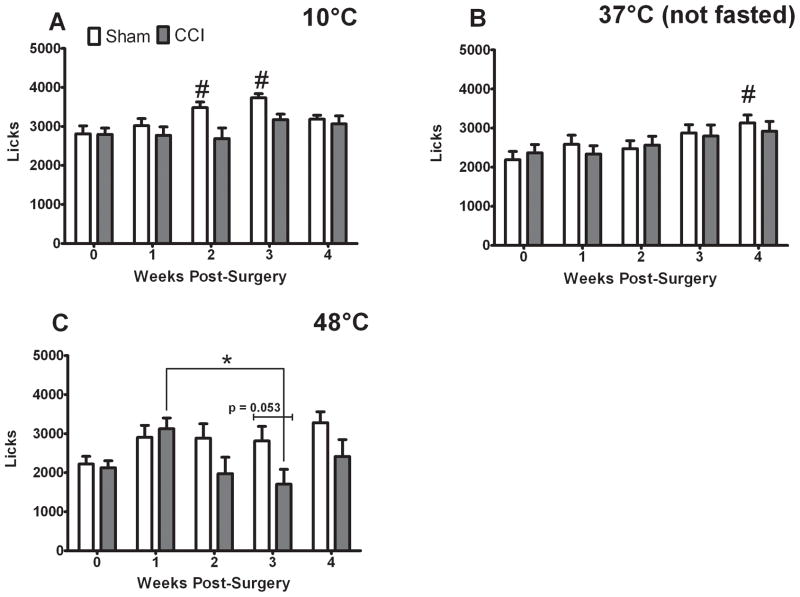
CCI treatment did not significantly decrease reward licks following surgery (Fig. 3). The only significant treatment effect (F = 10.00 p < 0.05) and significant interaction (F = 3.633 p < 0.05) observed for licks was with 48°C stimulation (Fig. 3C). A trend towards significantly decreased licks in the CCI treated rats compared to shams was observed at three weeks post-surgery (p = 0.053, Fig. 3C). While we did observe a significant effect of time on licks for all stimuli (10°C: F = 6.014 p <0.001, 37°C: F = 3.454 p < 0.05, 48°C: F = 4.854 p < 0.05), this effect was due to changes in the licking of sham-treated rats at 10 and 37°C (Fig. 3A, B) and a difference in licks between one and three weeks post-CCI (Fig. 3C). Such increases in licks occur under naïve, non-painful states.
Figure 3.
CCI does not inhibit reward licks in the presence of 10 and 37°C stimuli, and only slightly reduces licks in the presence of 48°C stimulation. Licks in the presence of 10°C (A), 37°C (B) were affected by time only in the sham-treated rats (# indicates significant difference from baseline and the indicated time points, ANOVA). Licks in the presence of 48°C (C) exhibited a significant treatment and time interaction. Post-hoc tests revealed that CCI-treated rats licked more at one week post surgery than at three weeks post-surgery (indicated by * over lines, ANOVA). No post-hoc differences were observed between CCI and sham-treated rats’ licks, although there was a trend at three weeks post-surgery (p =0.053, t-test). P< 0.05 and all data are mean ± SEM.
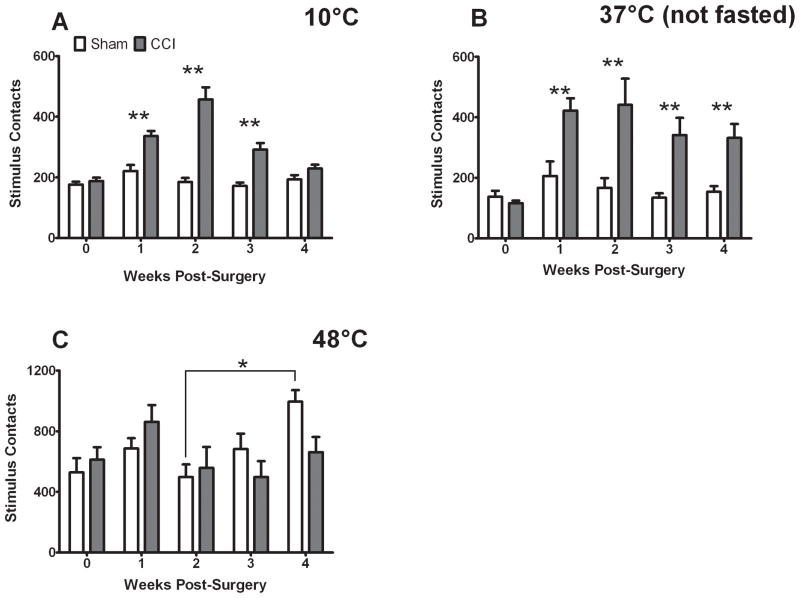
In contrast, facial contacts with the stimulus were substantially increased by CCI treatment in the presence of 10 and 37°C stimulation for up to three (10°C) or four (37°C) weeks post-surgery (Fig. 4A,B). There were significant effects of treatment (10°C: F = 40.43 p<0.001, 37°C: F = 31.164, p<0.001), significant effects of time (10°C: F = 7.854 p<0.001, 37°C: F = 5.153 p < 0.05), and their interaction (10°C: F = 6.522 p<0.001, 37°C: F = 2.884 p < 0.05) on facial contacts with the stimulus. For 48°C stimulation we observed only a significant effect of time on facial contacts with the stimulus (F = 2.69 p < 0.05), which was due to a temporal differences in contacts exhibited by sham-treated rats following surgery (Fig. 4C)
Figure 4.
CCI increases facial contacts with 10 and 37°C stimuli, but not a 48°C stimulus. Facial contacts with 10°C (A) and 37°C (B), were significantly increased in CCI-treated rats relative to their own baseline and to their sham counterparts (indicated by **, ANOVA and t-test). Facial contacts with 48°C (C) were not significantly affected by CCI-treatment. Sham-treated rats contacted the 48°C stimulus significantly more frequently at four weeks post-surgery than they did at two weeks post-surgery (indicated by * over lines, ANOVA). P< 0.05 and all data are mean ± SEM.
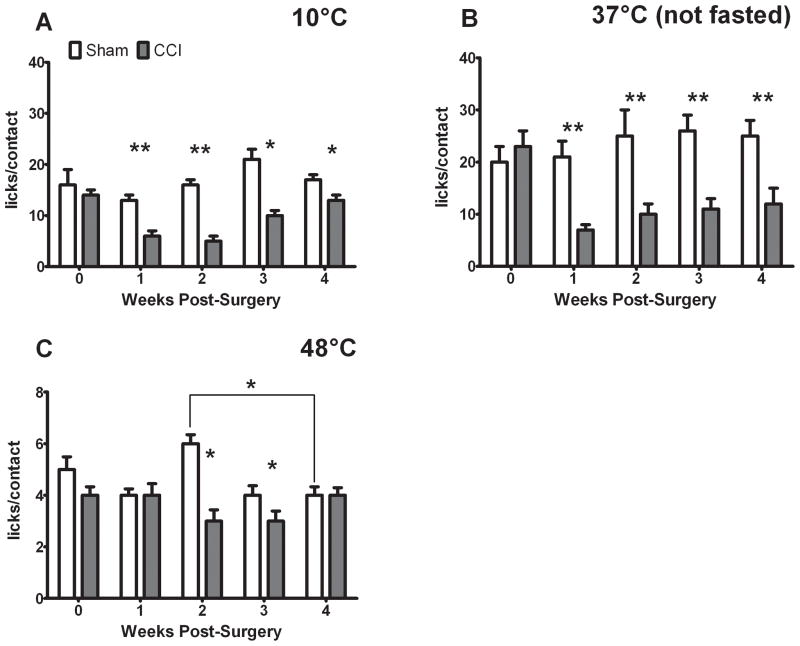
This increase in facial contacts with 10 and 37°C stimuli is reflected by a corresponding decrease in the pain indices (licks/contact) of CCI-treated rats postoperatively, relative both to their own baseline and to sham pain indices (Fig. 5A, B). This was indicated by a significant treatment effect for both of these stimuli on pain index (10°C: F = 29.086, 37°C: F = 30.801, p<0.001 for both), a significant effect of time on 10°C pain index (F = 5.919, p<0.001) and a significant interaction for both 10 and 37°C stimulation (10°C: F = 5.692, 37°C: F = 3.59, p < 0.05). For 48°C stimulation, CCI-treated rats’ pain index was lower than their sham counterparts at two and three weeks post-surgery (Fig. 5C), but never differed from their own pre-operative pain index. This was indicated by a significant effect of treatment (F = 19.86, p<0.001), no significant effect of time, and a significant interaction (F = 3.979, p <0.05).
Figure 5.
CCI reduces pain indices (licks/contact) with 10 and 37°C stimulation, but does not reduce pain index from baseline with 48°C stimulation. CCI-treated rats exhibit reduced pain-indices with 10°C stimulation (A) compared to both their own baseline and their sham-counterparts at one and two weeks post-surgery (indicated by **, ANOVA and t-test). At three and four weeks post-surgery the pain indices of CCI-treated rats were still lower than their sham-counterparts (indicated by *, t-test). CCI-treated rats also exhibited reduced pain indices at all post-operative time points with 37°C stimulation (B) compared to both their own baseline and their sham-counterparts (indicated by **, ANOVA and t-test). There was no effect of sham treatment for either of these temperatures. In contrast, CCI treated rats’ post-operative pain indices with 48°C stimulation (C) did not differ from their pre-operative baseline, only with their sham-treated counterparts at two and three weeks post-surgery (indicated by *, t-test). Sham-treated rats also exhibited significantly greater pain indices at two weeks post-surgery than at four weeks (indicated by * over lines, ANOVA). There was a significant effect between treatment groups at 48°C, but there was a significant difference between sham pain indices at 2 and 4 weeks post-treatment (indicated by *). P < 0.05 and all data are mean ± SEM.
Taken together, these finding indicate that following CCI-treatment, rats contact the 10 or 37°C stimulus more frequently after surgery in order to lick the milk reward at the same frequency as they were able to before injury. This strategy of contacting the stimulus more briefly and frequently is one that we have observed previously as temperature increases from 45°C under naïve conditions or under inflammatory conditions (Neubert et al. 2005), which indicates pain. This strategy was not observed in sham-treated rats with 10 or 37°C stimulation or in CCI-treated rats with 48°C stimulation. In contrast, 48°C is already quite painful in the absence of injury (note that baseline pain indices at 48°C are equivalent or slightly less than post-CCI pain indices for 10 and 37°C, Fig. 5C versus Fig. 5A,B). Thus, we may have a floor effect when combining this painful stimulus with a fasted state and salient reward.
Effect of Surgical Treatment on Nerve Inflammation and Injury
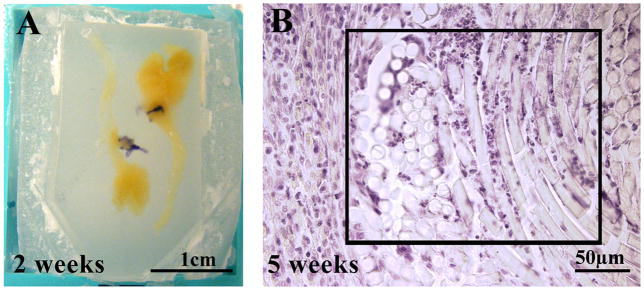
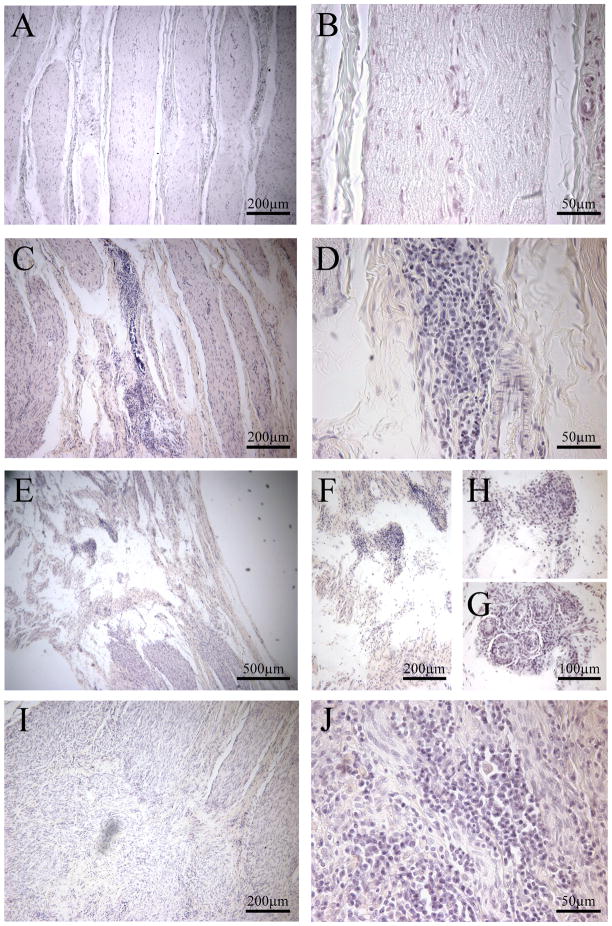
Nerves from CCI- and sham-treated rats were assessed for degree of inflammation and nerve injury. The knots of the vicryl ligatures were grossly visible only around nerves taken two weeks post-surgery (Fig. 6A); at all later time points suture was identified microscopically in nerves as late as 7 weeks post-surgery (Fig. 6B). A treatment-blind investigator indicated that two sham-treated nerves samples had microscopic signs of suture. One of these samples came from a rat that consistently displayed head tilting and exhibited reductions in pain indices with all stimuli. This individual was therefore excluded from the sham treatment group for all analysis. Nerves taken at 2–7 weeks post-surgery exhibited mild to moderate tissue loss (Fig. 7C, E). Within these zones of missing nervous tissue, infiltrating immune cells were observed (Fig. 7D, F, H, I, J) and foreign body reactions (Fig. 7G), in some cases. In contrast, nerves taken at 12 weeks post-surgery exhibited smaller, isolated areas of tissue loss, and fewer discrete loci of immune cell infiltration. As a negative control, nerves from two naive individuals were consistently declared “healthy” by the treatment-blinded investigator (Fig. 7A, B).
Figure 6.
Presence of suture and assessment of nerve inflammation and injury in surgically treated infraorbital trigeminal nerves. Examples of grossly visible suture (violet fibers) around nerve removed two weeks post-CCI (A) and microscopically visible suture (B) (striate whirls of transparent material with infiltrating lymphocytes, in boxed area) within a nerve removed at five weeks post-CCI (hematoxylin and eosin).
Figure 7.
Exemplars of nerves with injury and inflammation post-surgery. A-B are images of an untreated nerve, with no injury or inflammation. C-J are examples of varying degrees of injury and inflammation apparent in chronic constriction injured nerves taken 2–7 weeks post-surgery. A, C, E, I (left panels) are lower magnification to illustrate nerve injury, as indicated by the infiltration of chronic inflammatory cells, lymphocytes. B,D, F-H, J (right panels) are higher magnification to show the degree of inflammation. G depicts giant cells/foreign body reaction and inflammatory cells. All nerves were stained with hematoxylin and eosin.
Effect of Surgical Treatment on TRPV1 and TRPM8 Immunohistochemistry
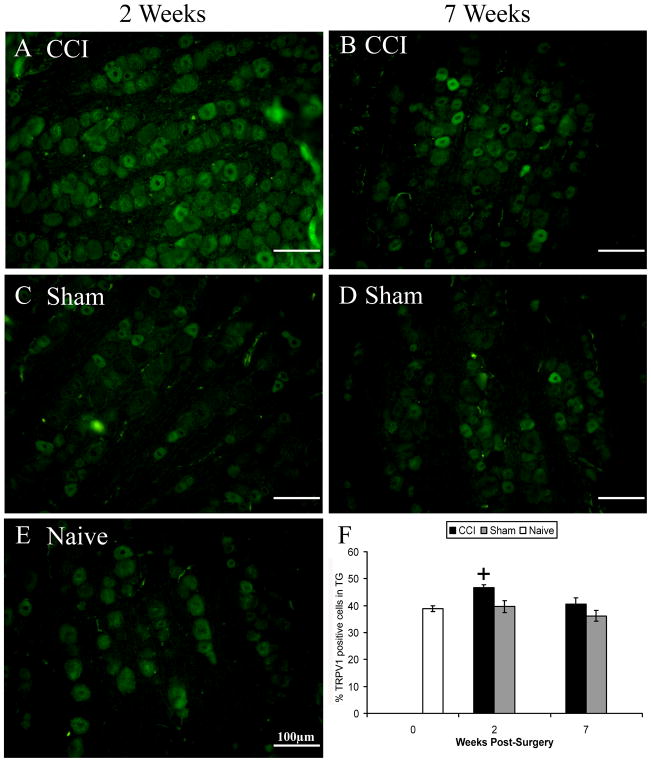
Trigeminal ganglia from CCI- and sham-treated rats were taken at two and seven weeks post-surgery and sections were either stained with antibodies against TRPV1 or TRPM8. Assessment of immunoreactivity was restricted to the area innervated by the infraorbital region of the trigeminal nerve. We observed the greatest percentage of TRPV1 positive cells in ganglia taken at two weeks post-CCI, as compared to all other treatment groups, even ganglia taken seven weeks post-CCI (Fig. 8).
Figure 8.
Increased percentage of TRPV1 immunoreactive cells in trigeminal ganglia of CCI-treated rats. A-E are exemplars of TRPV1 immunoreactivity in different treatment groups, as indicated, at two (left) or seven (right) weeks post-surgery. There was a significant increase in TRPV1 immunoreactivity in CCI-treated rats associated with two weeks post-surgery, but not seven weeks (F). + indicates significant difference from all other groups. Data are mean ± SEM.
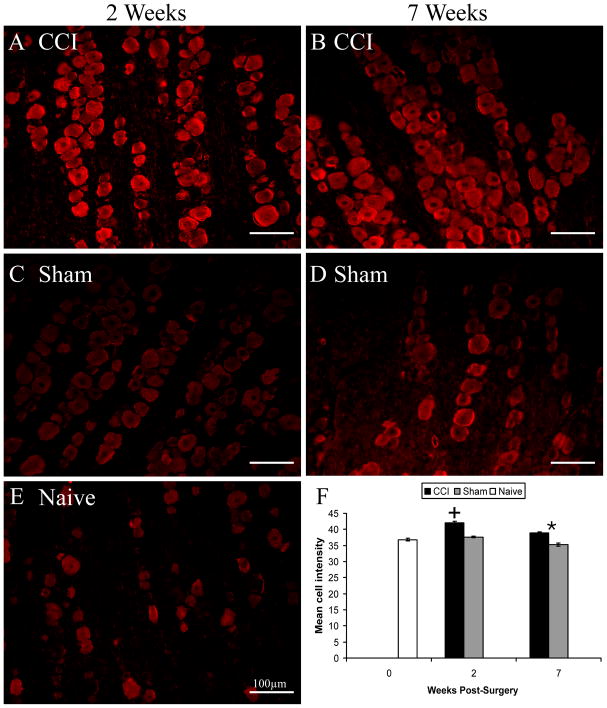
While no change in the percentage of TRPM8 positive cells was noted, the intensity of TRPM8 staining was greatest in ganglia taken two weeks post-CCI compared to all other treatment conditions (Fig. 9). TRPM8 staining was also more intense seven weeks following CCI as compared to sham treatment, but was not greater than naïve levels (Fig. 9). Taken together, these findings indicate that TRPV1 expression is detectable at two weeks following CCI treatment in cells that do not normally express this channel, but that this is also reversible. TRPM8 expression is also increased following CCI, but only in cells that normally express this protein, as indicated by immunohistochemistry. This peak in TRPM8 staining also occurs at two weeks post-surgery.
Figure 9.
Increased intensity of TRPM8 immunoreactivity in trigeminal ganglia of CCI-treated rats. A-E are exemplars of TRPM8 immunoreactivity in different treatment groups, as indicated, at two (left) or seven (right) weeks post-surgery. There was a significant increase in TRPM8 staining intensity in CCI-treated rats at both postoperative time points (F). + indicates significant difference from all other groups, * indicates significant difference between the two surgical treatments at seven weeks post-surgery. Data are mean ± SEM.
Discussion
We have demonstrated that rats receiving CCI of the trigeminal nerves exhibit pronounced aversive behaviors (head tilting and wiping at the stimulus) regardless of the temperature. It is likely that these nocifensive behaviors are in response to the sensation evoked by brushing the skin across the metal thermodes. This suggests the presence of dynamic mechanical allodynia, a common feature of neuropathic pain (Bowsher, 2005, Lang et al., 2006, Samuelsson et al., 2007). The combination of various temperatures in addition to mechanical contact may further facilitate or inhibit pain in this assay. Cold stimulation at 10°C seems to facilitate pain and alter behavior in the operant task, whereas 24°C stimulation does not (Kumada et al., 2012). Interestingly, neutral stimulation (37°C) and heat (45°C) stimulation (Kumada et al., 2012) were also pain facilitating, whereas 48°C stimulation did not further decrease the operant pain index relative to baseline. The latter finding was surprising, given that heat hyperalgesia, as indicated by robust decrease in withdrawal latency from radiant heat, has been associated with unilateral CCI of the trigeminal nerve (Imamura et al., 1997).
It is clear that at 10°C, when fasted, and at 37°C, when not fasted, CCI-treated rats adopt the same strategy that naïve rats execute for hot stimuli (more frequent, short contacts) to maintain the same level reward licks that they achieved pre-operatively. Kumada and colleagues also observed this effect following CCI treatment with 45°C stimulation following trigeminal CCI, when compared to baseline or to the behavior of sham treated rats (Kumada et al., 2012). In contrast, this strategy appears to already be maximally involved at 48°C (i.e. there is a floor effect for the pain index). The slight decrease in licks with 48°C suggest that this strategy of maximizing milk consumption may be less effective for CCI treated rats at two and three weeks post-surgery, but this decrease was not significant relative to their baseline licks. Perhaps if the animals were tested without fasting, a greater difference may be observed in the behavior of CCI- and Sham-treated rats with this stimulus. These decreases in pain indices and persistent aversive displays coincided with the presence of inflammation and injury in CCI-treated nerves. This is in agreement with studies that have characterized the time course of immune cells infiltration into the nerve following sciatic (Sommer and Schäfers, 1998) or mental (trigeminal) nerve injury (Lee and Zhang, 2012). Macrophages are highly present in injured nerves as late as four weeks, but gone by 88 days (13 weeks) after the injury (Lee and Zhang, 2012). These macrophages have the capacity to secrete cytokines, which could have sensitizing effects on various channels found on sensory axons within the nerve, including TRPV1 (Cheng and Ji, 2008).
Additionally, we also observed an increased percentage of TRPV1 expression and increased intensity of TRPM8 staining in the trigeminal ganglia associated with CCI treatment. These changes were maximal at two weeks-post surgery, corresponding to allodynia at 10 and 37°C. These findings regarding enhancement of TRPV1 and TRPM8 expression following neuropathic injury are in agreement with several other studies (de Leeuw et al., 2005, Ma et al., 2005, Wilson-Gerwing et al., 2005, Proudfoot et al., 2006, Biggs et al., 2007, Su et al., 2012).
Trigeminal CCI has been associated with cold allodynia as assessed by a cooling spray (Chichorro et al., 2006), but the exact range of temperatures that evoke this response is not quantifiable by this method. Our findings with respect to 10°C stimulation are in agreement with other studies involving operant evaluation of sciatic nerve injury (Vierck et al., 2005, Jabakhanji et al., 2006, Walczak and Beaulieu, 2006). However, it is surprising that cold allodynia is not apparent with 24°C stimulation in the operant assay. It is possible that this discrepancy may be due to competing activities of non-nociceptive and nociceptive TRPM8-expressing populations. Xing and colleagues have previously observed that sciatic CCI increases the percentage of cells that express both TRPV1 and TRPM8 (Xing et al., 2006, Xing et al., 2007), suggesting an increase in nociceptive TRPM8 cells. Menthol treatment (i.e. TRPM8 activation) can alleviate mechanical allodynia associated with sciatic CCI (Su et al., 2012). It is possible that activation of a non-nociceptive population of TRPM8-expressing cells by 24°C stimulation inhibits mechanically-evoked nociceptive input. In contrast, with 10°C stimulation, the activity of cold and mechanically responsive nociceptors may produce a net activation, facilitating pain transmission. Further work is needed to confirm this hypothesis and evaluate the role of other channels in this phenomenon.
Another surprising finding was the prolonged allodynia to neutral (37°C) stimulation. Heat hyperalgesia following trigeminal chronic constriction has been demonstrated (Imamura et al., 1997), but there is not much information regarding evoked behavioral responses near body temperature. At least one other group reported enhanced reflexive withdrawal from a 38 °C stimulus in the streptozotocin model of diabetic neuropathy (Beyreuther et al., 2006) and in a model of chemotherapeutic pain (Beyreuther et al., 2007), but mechanical allodynia was also associated with these findings (Beyreuther et al., 2006; Beyreuther et al., 2007). Changes in TRPV1 activation threshold and distribution of this channel may contribute to the allodynia observed with 37°C stimulation. The activation curve of TRPV1 can be left shifted by acidosis associated with inflammation and by inflammatory mediators (Cheng and Ji, 2008). This means that TRPV1 can become active at body temperature. TRPV1 expression is also increased in medium diameter cells following mental (trigeminal) nerve transection, possibly representing a phenotypic switch of normally non-nociceptive cells to nociceptive (Kim et al., 2008). Thus, in the context of neuropathic injury, ectopic nociceptor activity associated with body temperature, combined with mechanical nociceptor activity, could provide a net facilitation of pain. While this may explain the allodynia we observed at 37°C, it should also be noted that rats were not fasted when testing this stimulus. Diminished reward salience in the unfasted state combined with pain could also contribute to poor reward acquisition in the operant task. However, this seems to be unlikely given the fact that licks are similar pre- and post-CCI.
In addition to changes in the ganglia, other levels of nociceptive processing may contribute to our behavioral findings. The lack of effect on heat-mediated operant behaviors could also be related to the convergent processing of the affective, cognitive, and intensity aspects of painful stimulation. In patients with unilateral trigeminal neuropathic pain, cold and brush stimulation, but not heat, increased activity in the thalamus and anterior cingulate cortex relative to stimulation of the unaffected side (Becerra et al., 2006). This matched their psychophysical data indicating significant mechanical and cold allodynia, but no increased sensitivity to heat (Becerra et al., 2006). This lack of change in human pain rating is similar to our current findings, indicating that this is a translatable tool for evaluating facial neuropathic pain.
Despite the apparent aversive behavioral displays made by CCI rats postoperatively, they still lick the milk reward as much as the sham counterparts and their own pre-operative levels. This would suggest that the salience of the appetitive milk reward has not changed, despite a state of ongoing pain that clearly requires that rats change their strategy to achieve reward. Recent work has suggested that neural circuitry related to reward and aversion are involved in modulating pain sensitivity, and may be involved in the generation of chronic pain (Becker et al., 2012). Changes in the activation of reward-aversion circuitry may also be involved in the co-morbidity of chronic pain conditions with depression and anxiety (Borsook et al., 2007). This operant pain assay may provide a tool for examining the interaction between factors like stress and injury that modulate both pain and reward sensitivity in complement of research with human subjects.
Conclusion
We show here that operant responses to facial cold and neutral stimulation are in agreement with previous studies using reflex, unlearned, and operant behavioral assessment of neuropathic allodynia in the hindpaw. In contrast, operant responses to facial heat stimulation are not as inhibited by neuropathic injury. In addition to changes in operant behavior, we also observed aversive behaviors consistent with a nocifensive response against mechanical allodynia. These behavioral changes are accompanied by changes in both TRPV1 and TRPM8 expression at two weeks post-surgery that may underlie allodynia observed at this time point. These findings indicate that operant assessment of facial pain can provide insights regarding the interaction between nociception and motivating factors that shape pain-related behavior. This may lead to a better understanding of the multifactorial processes that lead to intractable chronic pain.
Supplementary Material
Highlights.
An operant assay was used to characterize trigeminal neuropathic pain.
Allodynia was evident (10 and 37°C), but heat hyperalgesia (48°C) was not.
Inflammatory cell infiltration and nerve injury coincided with behavioral changes.
The percentage of TRPV1+ cells and intensity of TRPM8 staining increased in TGs
The behavior matches clinical findings, indicating translatability of the assay.
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research grant 5R21DE016704-02.
Abbreviations
- CCI
Chronic Constriction Injury
- TRPV1
Transient Receptor Potential Channel Vanilloid 1
- TRPM8
Transient Receptor Potential Channel Melastatin 8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal Neuropathic Pain Alters Responses in CNS Circuits to Mechanical (Brush) and Thermal (Cold and Heat) Stimuli. The Journal of Neuroscience. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci Lett. 2012 Mar 14; doi: 10.1016/j.neulet.2012.03.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Benoliel R, Eliav E, Tal M. No sympathetic nerve sprouting in rat trigeminal ganglion following painful and non-painful infraorbital nerve neuropathy. Neuroscience Letters. 2001;297:151–154. doi: 10.1016/s0304-3940(00)01681-5. [DOI] [PubMed] [Google Scholar]
- Beyreuther B, Callizot N, Stöhr T. Antinociceptive efficacy of lacosamide in a rat model for painful diabetic neuropathy. European Journal of Pharmacology. 2006;539:64–70. doi: 10.1016/j.ejphar.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Beyreuther BK, Callizot N, Brot MD, Feldman R, Bain SC, Stöhr T. Antinociceptive efficacy of lacosamide in rat models for tumor- and chemotherapy-induced cancer pain. European Journal of Pharmacology. 2007;565:98–104. doi: 10.1016/j.ejphar.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, Robinson PP. Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. European Journal of Pain. 2007;11:192–201. doi: 10.1016/j.ejpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. European Journal of Pain. 2007;11:7–7. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bowsher D. Dynamic mechanical allodynia in neuropathic pain. Pain. 2005;116:164–165. doi: 10.1016/j.pain.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Caudle R, King C, Nolan T, Suckow SK, Vierck CJ, Neubert JK. Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin. J Pain. 2010;11:838–846. doi: 10.1016/j.jpain.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J-K, Ji R-R. Intracellular Signaling in Primary Sensory Neurons and Persistent Pain. Neurochemical Research. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichorro JG, Zampronio AR, Petto Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: Reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MKH, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochemical and Biophysical Research Communications. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Albuquerque R, Okeson J, Carlson C. The contribution of neuroimaging techniques to the understanding of supraspinal pain circuits: Implications for orofacial pain. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2005;100:308–314. doi: 10.1016/j.tripleo.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Experimental Brain Research. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources US. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [DOI] [PubMed] [Google Scholar]
- Jabakhanji R, Foss JM, Berra HH, Centeno MV, Apkarian AV, Chialvo DR. Inflammatory and neuropathic pain animals exhibit distinct responses to innocuous thermal and motoric challenges. Mol Pain. 2006;2 doi: 10.1186/1744-8069-1182–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayserl V, Latrémolièrel A, Hamonl M, Bourgoinl S. N-methyl-d-aspartate receptor-mediated modulations of the anti-allodynic effects of 5-HT1B/1D receptor stimulation in a rat model of trigeminal neuropathic pain. European Journal of Pain. 2011;15:451–458. doi: 10.1016/j.ejpain.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kim SM, Kim J, Kim E, Hwang SJ, Shin HK, Lee SE. Local application of capsaicin alleviates mechanical hyperalgesia after spinal nerve transection. Neuroscience Letters. 2008;433:199–204. doi: 10.1016/j.neulet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Kumada A, Matsuka Y, Spigelman I, Maruhama K, Yamamoto Y, Neubert JK, Nolan TA, Watanabe K, Maekawa K, Kamioka H, Yamashiro T, Kuboki T, Oguma K. Intradermal injection of Botulinum toxin type A alleviates infraorbital nerve constriction-induced thermal hyperalgesia in an operant assay. Journal of Oral Rehabilitation. 2012;39:63–72. doi: 10.1111/j.1365-2842.2011.02236.x. [DOI] [PubMed] [Google Scholar]
- Lang PM, Schober GM, Rolke R, Wagner S, Hilge R, Offenbächer M, Treede R-D, Hoffmann U, Irnich D. Sensory neuropathy and signs of central sensitization in patients with peripheral arterial disease. Pain. 2006;124:190–200. doi: 10.1016/j.pain.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Latrémolière A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M, Pohl M. Differential Implication of Proinflammatory Cytokine Interleukin-6 in the Development of Cephalic versus Extracephalic Neuropathic Pain in Rats. The Journal of Neuroscience. 2008;28:8489–8501. doi: 10.1523/JNEUROSCI.2552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang J. Heterogeneity of macrophages in injured trigeminal nerves: Cytokine/chemokine expressing vs. phagocytic macrophages. Brain, Behavior, and Immunity. 2012 Mar 25; doi: 10.1016/j.bbi.2012.03.004. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lipton J, Ship J, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Ma W, Zhang Y, Bantel C, Eisenach JC. Medium and large injured dorsal root ganglion cells increase TRPV-1, accompanied by increased [alpha]2C-adrenoceptor co-expression and functional inhibition by clonidine. Pain. 2005;113:386–394. doi: 10.1016/j.pain.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Neubert J, Rossi H, Pogar J, Jenkins A, Caudle R. Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors. Behavioral and Brain Functions. 2007;3 doi: 10.1186/1744-9081-1183-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Rossi HL, Malphurs W, Vierck JCJ, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural Brain Research. 2006;170:308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck JCJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Current Biology. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Rossi HL, Neubert JK. Effects of environmental enrichment on thermal sensitivity in an operant orofacial pain assay. Behavioural Brain Research. 2008;187:478–482. doi: 10.1016/j.bbr.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Vierck CJ, Caudle R, Neubert J. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Molec Pain. 2006;2 doi: 10.1186/1744-8069-1182-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson M, Leffler A-S, Johansson B, Hansson P. On the repeatability of brush-evoked allodynia using a novel semi-quantitative method in patients with peripheral neuropathic pain. Pain. 2007;130:40–46. doi: 10.1016/j.pain.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: Findings form functional imaging studies. Cell Mol Life Sci. 2009;66:375–390. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schäfers M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Research. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Su L, Wang C, Yu Y-h, Ren Y-y, Xie K-l, Wang G-l. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neuroscience. 2012;12 doi: 10.1186/1471-2202-1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua AJ, Johnson RD. Bilateral Chronic Constriction of the Sciatic Nerve: A Model of Long-term Cold Hyperalgesia. The Journal of Pain. 2005;6:507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Walczak J-S, Beaulieu P. Comparison of three models of neuropathic pain in mice using a new method to assess cold allodynia: The double plate technique. Neuroscience Letters. 2006;399:240–244. doi: 10.1016/j.neulet.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VMK. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.