Abstract
Current cancer therapies including cytotoxic chemotherapy, radiation and hyperthermic therapy induce acute proteotoxic stress in tumour cells. A major challenge to cancer therapeutic efficacy is the recurrence of therapy-resistant tumours and how to overcome their emergence. The current study examines the concept that tumour cell exposure to acute proteotoxic stress results in the acquisition of a more advanced and aggressive cancer cell phenotype. Specifically, we determined whether heat stress resulted in an epithelial-to-mesenchymal transition (EMT) and/or the enhancement of cell migration, components of an advanced and therapeutically resistant cancer phenotype. We identified that heat stress enhanced cell migration in both the lung A549, and breast MDA-MB-468 human adenocarcinoma cell lines, with A549 cells also undergoing a partial EMT. Moreover, in an in vivo model of thermally ablated liver metastases of the mouse colorectal MoCR cell line, immunohistological analysis of classical EMT markers demonstrated a shift to a more mesenchymal phenotype in the surviving tumour fraction, further demonstrating that thermal stress can induce epithelial plasticity. To identify a mechanism by which thermal stress modulates epithelial plasticity, we examined whether the major transcriptional regulator of the heat shock response, heat shock factor 1 (HSF1), was a required component. Knockdown of HSF1 in the A549 model did not prevent the associated morphological changes or enhanced migratory profile of heat stressed cells. Therefore, this study provides evidence that heat stress significantly impacts upon cancer cell epithelial plasticity and the migratory phenotype independent of HSF1. These findings further our understanding of novel biological downstream effects of heat stress and their potential independence from the classical heat shock pathway.
Keywords: Heat shock factor 1, Cell migration, Epithelial–mesenchymal transition, Proteasome inhibition, Heat shock
Introduction
The characteristic intrinsic properties of tumour cells as well as the tumour microenvironment lead to a significant and chronic disruption of protein homeostasis within the cancer cell. Intrinsic features of the tumour cell such as aneuploidy, oxidative stress, metabolic stress and high levels of mutant protein expression, combined with tumour microenvironmental stressors that can include hypoxia, nutrient deprivation and acidosis, generate high levels of misfolded proteins (Xie and Huang 2003; Dai et al. 2012). Furthermore, other forms of proteotoxic stress can occur acutely upon the cancer cell via the administration of cancer treatments such as cytotoxic chemotherapy, radiation treatment and hyperthermic therapy (Rylander et al. 2005). The enhanced proteotoxic stress profile generated by these treatments, combined with an inability of the cancer cell to resolve excessive disruptions to protein homeostasis, is one mode by which these therapies can bring about cancer cell death. Utilisation of ‘proteotoxic stress overload’ as an inducer of cancer cell death is the rationale for the use of proteasome inhibitors such as Velcade®, in the treatment of a variety of cancer types (Neznanov et al. 2011; Workman and Davies 2011; Dou and Li 1999). However, while tumour cells show increased sensitivity to enhanced proteotoxic stresses, undergoing cell death more readily than non-transformed cells, tumour cells that persist following these treatments have been shown to characteristically exhibit a more advanced malignant phenotype and may prove problematic in the event of tumour relapse (Blagosklonny 2005a).
A major obstacle to effective cancer therapy is tumour recurrence, with the subsequent emergence of tumour cells that are significantly less responsive to treatment, often exhibiting enhanced aggressive phenotypes (Blagosklonny 2005a, b). The importance of determining the underlying mechanisms that enable tumour recurrence and their refractory nature to treatments is exemplified by the fact that clinical response to therapy often does not correlate with extended patient overall survival in advanced cancers (Huff et al. 2006; Blagosklonny 2005b). Recent studies have shown that in addition to activating pathways that confer enhanced cell survival, various forms of proteotoxic stress can promote properties associated with a more aggressive cell phenotype such as enhanced cell migration, invasion and epithelial-to-mesenchymal transition (EMT; Cannito et al. 2008; Mak et al. 2010; Zhong et al. 2011; Chakraborty et al. 2010; Tamminen et al. 2012; Forsyth et al. 2010). These studies suggest that in the likely event that therapies such as hyperthermic chemotherapy, radiation therapy and chemotherapeutics are not sufficient in causing tumour cell death, the proteotoxic stress undergone by surviving cells may actively progress these cells towards more aggressive phenotypes. Indeed, inhibition of angiogenesis that promotes tumour hypoxia has been shown to not only have both anti-tumour effects but can also promote tumour progression and metastasis (Ebos et al. 2009; Paez-Ribes et al. 2009). These findings emphasise proteotoxic stress as a potent mediator of cancer progression and highlight the importance of elucidating the extent to which pathways activated by proteotoxic stress contribute to aggressive tumour phenotypes. Whether the activation of stress pathways such as the heat shock response (HSR) or the unfolded protein response (UPR) promote aggressive tumour phenotypes still remains largely unknown.
The HSR is a major cellular stress pathway that is activated in response to increased levels of misfolded proteins. Activation of the HSR results in the increased expression of a family of molecular chaperone proteins known as the Heat Shock Proteins (HSPs). HSPs function to ensure the correct conformation of cellular proteins and during stress, promote cell survival through maintaining protein homeostasis and inhibiting apoptosis (Samali and Cotter 1996; Jolly and Morimoto 2000). Due to the general ‘stress phenotype’ of tumour cells, they have been shown to have a higher dependency upon HSP function, reflected by the increased expression levels of HSPs in numerous forms of human cancer (Khalil et al. 2011; Ciocca and Calderwood 2005; Dai et al. 2012).
Heat shock factor 1 (HSF1) is the ‘master’ transcriptional regulator of HSPs and essential for stress-induced activation of HSP genes during the HSR (McMillan et al. 1998; Anckar and Sistonen 2011; Xiao et al. 1999). Both increased levels of HSF1 expression and activation have been correlated with more aggressive forms of human cancer, and HSF1 has consequently been proposed as an attractive therapeutic target for cancer (Whitesell and Lindquist 2009; Santagata et al. 2011; Dai et al. 2012). Moreover, recent studies have revealed that HSF1 regulates transcriptional targets that extend well beyond the induction of classical HSP genes, these include IL-6, MDR-1, NFATc2 and CXCL8/IL-8, with gene expression microarray and ChIP analysis providing evidence for many more (Maity et al. 2011; Singh et al. 2008; Vilaboa et al. 2000; Page et al. 2006; Hayashida et al. 2010; Trinklein et al. 2004). Consistent with its broad transcriptional landscape, HSF1 is known to have a diverse range of physiological functions, including the modulation of cell migration, inflammatory and metabolic pathway regulation (Ianaro et al. 2001; O’Callaghan-Sunol and Sherman 2006; Dai et al. 2007; Xiao et al. 1999). Hyperthermic, radiation therapy and many chemotherapeutics are known to be potent activators of both HSF1 and the HSR (Nikfarjam et al. 2005; Rylander et al. 2005); however, whether activation of HSF1 during the administration of these therapies confers tumour cells with enhanced malignant properties in addition to increased survival is currently unknown.
This study seeks to investigate whether increased proteotoxic stress induced by heat shock or proteasome inhibition can modulate cancer cell migration and epithelial plasticity through the activation of HSF1.
Materials and methods
Cell culture, heat shock and drug treatments
Both the lung A549 and the breast MDA-MB-468 human adenocarcinoma cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10 % FBS (Thermo Scientific) and 1 % antimycotics/antibiotics (Gibco cat. 15240–062). Cells were maintained at 37 °C, 5 % CO2 in a humidified environment. Heat shock was performed by immersion into a water bath pre-heated in advance to achieve a stable temperature of 42 °C. Growth media was replaced with media warmed to 42 °C and the plates or flask were sealed with parafilm and immersed for the indicated period of time. Media was then replaced with growth media at 37 °C and incubated under standard growth conditions for the indicated recovery time. MG132 (Calbiochem) was resuspended in DMSO. Following MG132 treatment, cells were rinsed twice with phosphate buffered saline (PBS) and fresh media added after which the cells were incubated under standard growth conditions for the indicated recovery times.
Western blot analysis
Cells were rinsed with PBS and lysed with radioimmunoprecipitation buffer containing phosphatase and protease inhibitor cocktails (Sigma). Samples were sonicated four times for 30 s in ice-cold water in a bench top sonicating waterbath (Thermoline PowerSonic405) and clarified by centrifugation. Protein quantification was performed using the BCA protein assay kit (Pierce) as per manufacturer’s instructions. Protein (10 μg) was loaded onto a 4–20 % Tris–glycine polyacrylamide gradient gel (NuSep), transferred for 120 min at 90 V (Hoefer apparatus) to Immobilon-P PVDF membrane (Millipore). Membranes were blocked in 3 % skimmed milk/TBS + 0.05 % tween (TBST) for 30 min and then antibodies incubated either overnight at 4 °C or 1-h room temperature. The following antibodies were used; N-cadherin (Cell signalling 4061), E-cadherin (BD-610181), pan-actin (Neomarkers MS-1295-P), HSPA1A (HSP70-1; AbCam ab47455), HSP47 (AbCam ab13510), vimentin (abcam ab71144), HSF1 (Enzo ADI-SPA-901 or Epitomics 2043-1), HSPB1 (HSP27; Enzo ADI-SPA-800), HSPH1 (HSP105; Santa Cruz sc-6241) and HSF1 pS326 (Epitomics 2092-1). Unbound primary and horseradish peroxidase (HRP)-conjugated secondary (Thermo Scientific) antibodies were removed by washing in TBST, then incubated in Super Signal West Pico Chemiluminescent Substrate (Pierce) for 7 min followed by exposure to film (GE healthcare or Fuji Film) or imaged using Syngene G-Box ChemiXL imaging system.
Microchemotaxis migration assay
The microchemotaxis assay was performed as previously described using 8 μm (A549) or 12 μm (MDA-MB-468) collagen IV-coated membranes (Sigma; Price et al. 2005; Kouspou and Price 2011). Briefly, cells were counted and seeded at equal numbers in a T75 flask (Nunc) 48 h prior to the assay to achieve a confluence of 50–70 % on the day of the assay. The proteotoxic stress (heat shock or MG132 treatment) was performed 21–24 h prior to the chemotaxis assay. Cells were lifted non-enzymatically by incubation for 15 min in 1× PBS + EDTA, washed three times with 0.1 % bovine serum albumin (BSA; Sigma) serum-free DMEM and then resuspended at equal concentrations (0.8–1.0 × 106cells/ml). The lower wells of the microchemotaxis chamber (Neuroprobe) were loaded with 10 ng/ml epidermal growth factor (EGF; BD cat. 354052) in 0.1 % BSA DMEM as the chemoattractant or 0.1 % BSA DMEM as the negative control. Cells were loaded into the upper wells of the apparatus and incubated for 3.5–4 h at 37 °C, 5 % CO2. The chamber was then disassembled, the membrane was removed and cells were fixed in 100 % methanol for 2 min, followed by staining in Quickdip I for 1 min and QuickDip II (Fronine) for 2 min; the membrane was then mounted onto a glass slide and non-migrated cells were wiped off with a wet tissue. Migrated cells were imaged at ×200 magnification on CKX41 microscope (Olympus) and counted using ImageJ software.
Quantitative RT-PCR
Following 4 h of recovery after heat shock, total RNA was isolated using the Qiagen RNeasy kit according to manufacturer’s instructions. Total RNA was quantified by spectrophotometer and 2 μg of RNA was reverse-transcribed using SuperScript III Reverse-Transcriptase (Invitrogen). cDNA (10 ng) was added to the qPCR reaction using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). Samples were loaded into Rotorgene 3000 with cycle conditions hold time: 2 min, 95 °C cycle (95 °C, 10 s; 60 °C, 15 s; 72 °C, 20 s). Data was analysed using the LinReg PCR software, as outlined in Ruijter et al. (2009). The following primers were used (Sigma): human CDH1 forward—TGCCCCCAGAGGATGACACCC, reverse—CCCCTGTGCAGCTGGCTCAA and Vimentin forward—AGGCGAGGAGAGCAGGATTTCTCTG, reverse—ATTGCTGCACTGAGTGTGTGCAA. These genes were normalised to the house keeping gene RPL32 forward—CAGGGTTCGTAGAAGATTCAAGGG, reverse—CTTGGAGGAAACATTGTCAGCGATC.
shRNAmir retroviral vectors and delivery
HSF1 (NM_005526.2)-targeted siRNA sequences were designed using ‘Designer of Small Interfering RNAs-DSIR’ (http://biodev.extra.cea.fr/DSIR/DSIR.html) and these sequences were used to generate shRNA through the subsequent use of RNAi Central (http://katahdin.cshl.org:9331/siRNA/RNAi.cgi?type=shRNA). The shRNAmir(2) (target region 956–976: AACCCATCATCTCCGACATCAC), shRNAmir(4) (target region 2010–2030: CAGGTTGTTCATAGTCAGAAT) and Scramble (TCTCGCTTGGGCGAGAGTAA) oligomers were cloned into the retroviral MSCV-LMP vector (Open Biosystems, Thermo Scientific). HEK293T cells were transiently transfected with pVpack-Ampho (Agilent Technologies) and LMP vectors using Lipofectamine LTX reagent (Invitrogen). The media was replaced after 16 and 24 h; later, the retrovirus-conditioned media was collected and filtered using a 0.45-μm filter. A549 cells in log-phase growth were transduced by adding virus-containing media for a period of 24 h with the addition of 10 μg/ml of polybrene. Cells were then grown without virus and transduced cells were selected based on green fluorescent protein (GFP) expression using FACS (Flowcore, Monash University); selection gates were chosen to equalise GFP fluorescence between knockdown and scramble controls.
Immunofluorescence and microscopy
A549 cells were cultured on 13-mm coverslips in a 24-well plate. Prior to fixation, cells were rinsed twice in PBS followed by addition of 4 % paraformaldehyde for 15 min at 37 °C. Cells were permeabilised with 0.1 % Triton-X for 10 min at room temperature (RT) and blocked with 10 % FBS/PBS for 30 min at RT. E-cadherin antibody (BD) was added at 1:1,000 dilution overnight at 4 °C. Unbound antibody was removed by washing with PBS and an alexa-fluor 488 conjugated anti-mouse secondary antibody (Invitrogen) at 1:2,500 dilution was added. DAPI (Invitrogen D1306) was included as a nuclear stain and Texas Red-Phalloidin (Invitrogen T7471) to stain actin. Cells were imaged on a Nikon C1 confocal microscope with ×400 magnification. Analysis of E-cadherin localisation was performed using ImageJ software; eight 2-day cross-sections per cell, with total 25 cells chosen at random for each sample from five different random fields were measured using ROIs selected based on actin staining to determine sites of cell junctions. Measurements were averaged and then normalised to the values obtained for the centre of the cell. All phase contrast images were taken on a Nikon Eclipse microscope at ×200 magnification.
Thermal ablation tumour treatment and analysis
Formalin-fixed specimens of thermally ablated colorectal liver metastases were examined by immunohistochemistry for heat shock effects. Thermal ablation (TA) of tumour metastases was carried out on a murine model of colorectal liver metastasis in CBA mice as reported previously (Nikfarjam et al. 2005). In brief, thermal ablation was performed with a diode laser 400-μm bare tip optical quartz fibre (D-6100-BF, Dornier MedTech Laser GmbH, Germany), applying 40 J of power per tumour (20 s at 2 W). Average tissue temperatures reach 65 °C adjacent to the fibre site without causing tissue charring. For the day 0 time point, the whole liver was removed immediately after TA application and samples collected. For other time points, the abdomen was closed with sutures and the animals allowed to recover until culled at specific time points following TA treatment. In control animals, a sham ablation was performed by inserting the probe into the tumour but with no activation of the probe being applied. For this study, changes in EMT markers were only investigated at 24 h after treatment. In a previous study, HSPA1A levels were found to peak at 24 h after TA treatment (Nikfarjam et al. 2005).
Immunohistochemistry
Formalin-fixed paraffin-embedded 4-μm-thick sections of tissue were deparaffinised and rehydrated using standard techniques. A polymer labelling kit was used for immunostaining according to the manufacturer’s instructions (Dako EnVision Plus, Dako). Endogenous peroxidases were blocked by incubation in 3 % hydrogen peroxide for 30 min at RT. Antigen retrieval for the detection of E-cadherin was performed by incubation in Citrate buffer (pH 6) at 99–100 °C then allowed to cool at RT for 20 min. Antigen retrieval for detection of Zeb1 was performed by incubation in Tris buffer (pH 7.4) at 99–100 °C then allowed to cool at RT for 20 min. Normal goat serum (20 %) was used to block non-specific binding. Tissue sections were incubated with respective primary antibodies, E-cadherin (sc-7870, 1:500) and Zeb1 (sc-25388, 1:200) (both antibodies obtained from Santa Cruz Biotechnology, ThermoFisher Scientific) for 1 h at 37 °C then overnight at 4 °C. For negative controls, sections were incubated with non-immune rabbit IgG only (DakoCytomation, Glostrup) at the same concentration as the primary antibody. Dako EnVision kit was then used containing goat anti-rabbit immunoglobulins (IgG) coupled with HRP (EnVision Plus, Dako, Australia) according to manufacturer’s instruction. Each incubation step was followed by two 5-min washes with TBS + 0.05 % Tween 20. Positive cells with intense staining were identified by incubation with diaminobenzidine solution for 5 min.
Statistical analysis
Data is presented as mean ± SD or SEM. Student’s t tests were conducted to determine whether the treatment group was statistically significant compared to controls. * p < 0.05, ** p < 0.01 and *** p < 0.001.
Results
Heat shock stimulates enhanced cell migration in the A549 and MDA-MB-468 cell lines
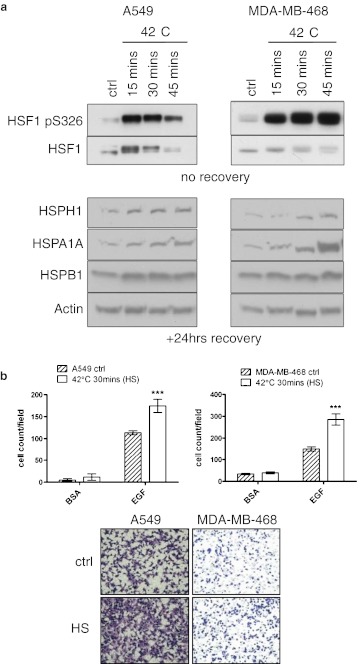
To determine the conditions in which the HSR is activated in both the lung A549 and the breast MDA-MB-468 human adenocarcinoma cell lines, cells were treated at 42 °C for 15, 30 and 45 min (Fig. 1a). Each condition efficiently activated HSF1 as detected by its increased phosphorylation status at serine 326, the shift in its band size and the increased expression of HSPs (Fig. 1a; Guettouche et al. 2005). Generally, higher levels of HSP induction correlated with longer treatment time. The treatment of both cell types at 42 °C for 30 min gave a strong induction of the HSR with very little morphological signs of cellular toxicity which was evident with longer treatment times. Therefore, a heat stress of 42 °C for 30 min with a 24-h recovery period was used in subsequent experiments.
Fig. 1.
Heat shock treatment increases the migratory capacity of cancer cell lines. a Activation of the HSR following heat shock in both the A549 and MDA-MB-468 cell lines as shown by HSF1 phosphorylation at serine326 and the induction of HSPH1 (HSP105), HSPA1A (HSP70-1) and HSPB1 (HSP27) expression. b Heat shock of A549 (n = 2) and MDA-MB-468 (n = 3) cells induces a significant increase in chemotactic cell migration following recovery. Chemoattractants include 0.1 % bovine serum albumin (BSA) as a background migration control and 10 ng/ml epidermal growth factor (EGF), ***p < 0.001
The migratory and chemotactic properties of a cancer cell closely reflect its invasive and metastatic potential (Condeelis et al. 2005; Wang et al. 2005). Therefore, to investigate the effects of heat shock upon mediating advanced cancer phenotypes, we examined the effects of this proteotoxic stress upon the migratory capacity of the cell lines. We utilised a standard 48-well microchemotaxis assay to determine cell migration in the A549 and the MDA-MB-468 cell lines after heat shock treatment and recovery. Although no alteration in the background migration of the cell lines was detected, as determined by use of the 0.1 % BSA control, upon heat shock, the chemotactic migration towards EGF of both cell types was greatly enhanced (Fig. 1b).
Heat shock stimulates mesenchymal properties in the A549 cell line
One mechanism by which carcinoma cells acquire a migratory phenotype is through the induction of an epithelial-to-mesenchymal transition (EMT). An EMT is defined by the loss of epithelial–cell polarity and cell–cell adhesion, coupled with enhanced cell migration, resistance to apoptosis, invasiveness and enhanced extracellular matrix remodelling properties (Zeisberg and Neilson 2009). In vitro, an EMT can be characterised through enhanced cell migration—a change from a cuboidal to elongated cell morphology and at the protein level—downregulation of epithelial markers such as E-cadherin and increased expression of mesenchymal makers such as Vimentin, HSP47, Zeb1 and N-cadherin (Thiery and Sleeman 2006; Zeisberg and Neilson 2009). E-cadherin is a cell adhesion molecule that is localised at the adherens junctions of epithelial cells and is responsible for cell–cell adhesion of neighbouring cells. Its peripheral cellular localization is a defining marker of epithelial morphology (Schmalhofer et al. 2009).
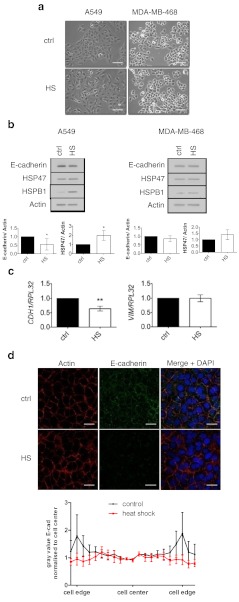
Therefore, we sought to characterise whether enhanced cell migration following heat shock was coupled to a shift towards a more mesenchymal cell phenotype indicative of an EMT. To achieve this, we examined cell morphological changes in conjunction with alterations in classical EMT marker expression and localization. Both the A549 and the MDA-MB-468 cell lines have an epithelial phenotype and have both been used as in vitro models for EMT (Lo et al. 2007; Kasai et al. 2005). Following a 24-h period of recovery from heat shock, A549 cells displayed reduced cell–cell contacts and had undergone scattering (Fig. 2a). In contrast to the A549 cells, little morphological change was observed in the MDA-MB-468 model (Fig. 2a). Consistent with the morphological alterations in the A549 cell, Western blot analysis revealed that the epithelial marker, E-cadherin, was significantly reduced following heat shock (Fig. 2b) and mRNA levels of E-cadherin were also observed to be decreased (Fig. 2c). Immunofluorescent staining of E-cadherin revealed that heat shock partially alters the localisation of E-cadherin with a trend towards reduced levels of the molecule being present at the cell periphery/cell–cell junction (Fig. 2d). In addition to E-cadherin, analysis of mesenchymal marker expression revealed that HSP47 levels were significantly increased following heat shock in the A549 model. However, no significant increase in N-cadherin or vimentin expression at the protein level (not shown) or at the level of gene expression was observed (Fig. 2c). Taken together, these results indicate a partial loss of epithelial properties in the A549 model following heat shock rather than a full transition towards a mesenchymal phenotype. In contrast to the A549 model, the induction of HSP47 and reduced E-cadherin expression following heat shock was not significant in the MDA-MB-468 model. Therefore, while both A549 and MDA-MB-468 cells exhibited enhanced cell migration upon heat shock, changes in cellular morphology and marker expression appeared to be cell line specific.
Fig. 2.
Heat shock induces alterations in EMT marker expression, localisation and cell morphology in A549 lung adenocarcinoma cells. a Cell scattering and change in morphology in heat-shocked A549 cell following 24 h of recovery, no distinct change is seen in the MDA-MB-468 cells, scale bar 100 μm. b Heat shock reduces the protein expression of the epithelial marker E-cadherin following 24 h of recovery in A549 cells. Densitometry represents the average fold change ±SD normalised to the loading control of actin (n = 3). c RT-qPCR showing downregulation of expression of the E-cadherin (CDH1) gene following 4 h of recovery. The graph represents fold change ±SD normalised to the RPL32 control gene (n = 3). d Heat shock induces altered levels and localisation of E-cadherin in A549 cells following 24-h recovery. The graph represents quantified E-cadherin distribution across the cell, average ±SEM, (n = 3), scale bar 25 μm, *p < 0.05, **p < 0.01
Thermal ablation treatment induces EMT in residual tumour fraction
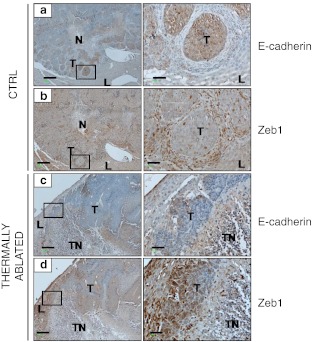
Thermal ablation is used in the clinic to destroy tumours by heat application. In a previous study, thermal ablation of murine colorectal liver metastases (CRCLM) was shown to induce the upregulation of HSPA1A in residual tumour cells that peaked at 24 h post-treatment (Nikfarjam et al. 2005). In the present study, we used archival tissues from a previous investigation (Fifis et al. 2011) to determine whether thermal ablation stimulated an EMT in the residual tumour fraction. Immunohistochemical analysis demonstrated that untreated tumours expressed high levels of E-cadherin (Fig. 3a) and minimal Zeb1 (Fig. 3b); however, strong Zeb1 expression was observed in infiltrating cells surrounding the tumour nodules (Fig. 3b inset). At 24 h post-TA treatment, the surviving tumour fraction had reduced E-cadherin expression (Fig 3c and inset), while Zeb1 (E-cadherin transcription repressor) expression was reciprocally increased within the cytoplasm and nuclei of surviving tumour cells following TA treatment (Fig. 3d and inset) indicating that the surviving residual tumour fraction had undergone an EMT.
Fig. 3.
Altered EMT marker expression in residual tumour fraction after TA treatment. Mice with colorectal liver metastases had two selected tumours thermally ablated. a and b Serial sections of formalin-fixed control tissues stained with antibodies against E-cadherin and Zeb1, respectively. c and d Serial sections of formalin fixed thermally ablated tumour tissues stained with antibodies to E- cadherin and Zeb1 respectively. N = tumour necrotic area, TN = thermally induced necrotic area, T = tumour, L = liver. Scale bar 250 μm, insert scale bar 50 μm
Heat shock induced changes in cell morphology; E-cadherin expression and enhanced migration are not dependent upon heat shock factor 1
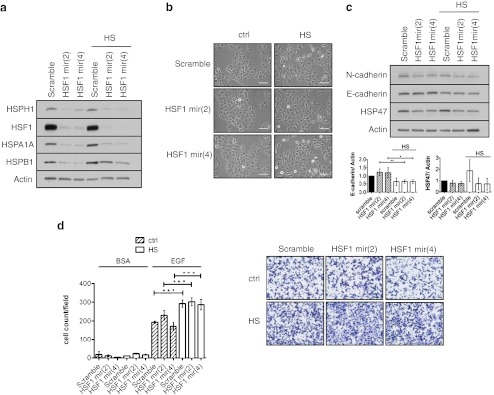
As HSF1 is the major transcriptional regulator of the HSR, we wanted to determine whether it was mechanistically involved in the heat stress-induced alterations to cell migration, morphology and EMT marker expression levels. To achieve this we utilised HSF1-targeted shRNAmir retroviral constructs that were stably expressed in the A549 cell line enabling the constitutive knockdown of HSF1 in these cells (Fig. 4a). Western blot analysis of HSF1 knockdown cells in comparison to control cells showed reduced protein expression of HSF1 transcriptional targets HSPH1 (HSP105), HSPA1A (HSP70-1) and HSPB1 (HSP27) under basal conditions as well as following heat shock (Fig. 4a). However, despite HSF1 knockdown cells being inhibited in their ability to efficiently induce HSP expression, administration of heat shock to the cells was still able to induce a scattered morphology (Fig. 4b) and reduce E-cadherin expression (Fig. 4c). HSP47 induction did not occur in response to heat shock in either of the knockdown cell lines, indicating that HSP47 expression was not linked to E-cadherin downregulation nor essential for the enhanced migratory phenotype induced by heat shock. Furthermore, despite the knockdown of HSF1, heat shock was still able to enhance chemotactic cell migration in the A549 cells (Fig. 4d). Thus, these results indicated that the changes to cellular morphology and the enhanced migratory capacity of the A549 cells induced by heat shock occur independently of HSF1.
Fig. 4.
Heat shock induces morphological change, altered EMT maker expression and enhanced chemomigration independent of HSF1. a Stable expression of targeted HSF1 shRNAmirs in A549 cells significantly reduces HSF1 protein expression and prevents induction of HSP expression following heat shock when compared to shRNAmir scramble control cells. b Heat shock induces cell dissociation independently of HSF1. c Heat shock induces altered expression of epithelial marker E-cadherin in HSF1 knockdown A549 cells, densitometry represents average fold change ±SD normalised to the scramble ctrl, (n = 3). d Heat shock with recovery enhances chemotactic cell migration in the A549 cell line independent of HSF1 (n = 2). Chemoattractants used are indicated at the top and were 0.1 % bovine serum albumin (BSA) and 10 ng/ml epidermal growth factor (EGF). *p < 0.05, **p < 0.01, ***p < 0.001
Proteotoxic stress through proteasome inhibition induces changes in cell morphology and enhanced migration that are heat shock factor 1 independent
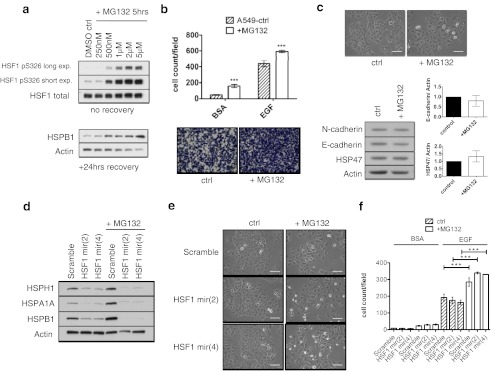
To further investigate a role of proteotoxic stress in modulating epithelial plasticity and the promotion of advanced cancer phenotypes, we examined the phenotypic effects induced by proteasome inhibition using the compound MG132 in the A549 cell line. Proteasome inhibition results in an accumulation of misfolded and dysfunctional proteins, resulting in HSF1 and HSR activation (Workman and Davies 2011). Previously, proteasome inhibition has been shown to be both inductive and inhibitory with respect to the EMT (Mak et al. 2010; Baritaki et al. 2009). The concentration of MG132 was initially optimised for a 5-h treatment period to enable a potent HSR to be induced. Treatment with 2 μM of MG132 over a 5-h period, resulted in a strong activation of HSF1 as indicated by increased phosphorylation of HSF1 at serine 326 and induction of HSPB1 expression after a 24-h recovery period (Fig. 5a). This treatment regime was effective in enhancing the chemotactic cell migration (Fig. 5b) and inducing a morphological shift towards a more mesenchymal phenotype of the parental A549 cells (Fig. 5c); however, no significant changes were observed in EMT marker expression (Fig. 5c). To investigate whether activation of HSF1 during proteasome inhibition mediated the observed phenotypic changes, we utilised the A549 HSF1 knockdown cells (Fig. 4a). As with heat shock, it was found that the HSF1 knockdown cells were unable to induce significant levels of HSP expression upon MG132 treatment (Fig. 5d). However, despite the effective knockdown of HSF1, MG132 was still able to induce the morphological changes towards a more mesenchymal phenotype (Fig. 5e) and enhance the migration of the A549 cell line (Fig. 5f). Therefore, as with heat stress, the phenotypic alterations induced by proteasome inhibition were independent of HSF1 in the A549 cell line.
Fig. 5.
MG132 treatment induces morphological changes and enhanced chemomigration independently of HSF1 in the A549 cell line. a Optimisation of MG132 treatment that induces a HSR in A549 cells. b Treatment of A549 cells with 2 μM MG132 for 5 h followed with 24-h recovery induces enhanced chemotactic cell migration (n = 2). c MG132 treatment induces disassociation of cells in standard tissue culture scale bar = 100 μm, with no significant difference in EMT marker expression (n = 2). d HSP expression is not induced by MG132 treatment in A549 cells expressing HSF1 shRNAmir. e Cell lines stably expressing HSF1 targeted shRNAmirs underwent morphological changes following MG132 treatment, scale bars 100 μm. f MG132 treatment with recovery enhanced chemotactic cell migration in the A549 cell line, independently of HSF1 (n = 2). Chemoattractants used in the experiment are labelled at the top and are 0.1 % bovine serum albumin (BSA) and 10 ng/ml epidermal growth factor (EGF). ***p < 0.001
Discussion
Activation of proteotoxic stress pathways such as the HSR have been proposed to not only support tumour cell survival following a variety of insults but may also be a major factor in promoting tumour progression (Dai et al. 2007; Rylander et al. 2005). Activation of the transcription factor, HSF1, is synonymous with HSR induction; however, in addition to its ability to confer enhanced survival of cells, HSF1 can also regulate many other cellular processes. This raises the potential that the HSR may stimulate a variety of cellular properties that are required for tumour recurrence and aggressive cancer cell phenotypes. A number of studies have shown that various forms of proteotoxic stress such as hypoxia, proteasome inhibition, ethanol treatment, endoplasmic reticulum (ER) stress, and oxidative stress-induced cell migration, invasion and EMT (Cannito et al. 2008; Chakraborty et al. 2010; Mak et al. 2010; Tamminen et al. 2012; Zhong et al. 2011). However, although cancer treatments such as hyperthermia treatment and pharmacological inhibition of the proteasome illicit proteotoxic stress and activate the HSR in tumour cells, their ability to promote aggressive cancer phenotypes has not been fully examined. Therefore, the aim of the current study was to determine whether proteotoxic stress, primarily in the form of heat stress, could promote advanced cancer phenotypes such as enhancing epithelial plasticity and cell migration. In addition, we wanted to determine whether HSF1 was an important mediator of the proteotoxic stress pathway effects upon the cancer cell phenotype.
Heat shock induces a wide variety of cellular responses including a dose-dependent induction of necrosis or apoptosis, stalled protein, RNA and DNA synthesis, and the induction of the HSR. Heat shock has also been shown to induce changes in cell shape, as well as altering cell membrane fluidity and membrane potential (Hildebrandt et al. 2002). However, to our knowledge, this is the first study in which heat shock has been shown to enhance chemotactic migration and epithelial plasticity.
The characterisation of cellular responses to heat stress have revealed both immediate and prolonged responses in terms of HSP induction and apoptotic signalling, as well as revealing that the dynamics of these cellular responses are dependent upon the intensity and duration of the heat stress. Previous investigations examining cellular responses to heat shock in vitro have utilised a variety of heat shock temperatures ranging from 41 to 45 °C and exposure times of between 15 min and 2 h (Garcia et al. 2012; Hildebrandt et al. 2002). Although a minimal heat shock was utilised in this study, represented by an exposure time of 30 min at 42 °C, a potent HSR was observed in the selected cell line models. In both the A549 and MDA-MB-468 cell lines, we observed enhanced cell migration after a 24-h period of recovery following heat shock. Although the relationship between the degree and length of heat shock exposure was not fully investigated in regards to cell migration, it was noted that a more prominent enhancement of cell migration was observed with an extended heat treatment of 1 h at 42 °C (not shown) in the A549 cell line. Even though this heat treatment induced higher levels of cellular toxicity, it did indicate that the migratory capacity of the cells was relative to the level of heat stress.
Heat shock was found to decrease E-cadherin steady-state protein levels, decrease its gene expression levels, as well as induce changes to the cellular morphology and enhance migration, indicative of the loss of epithelial cellular properties. Despite no detectable differences in cellular morphology or EMT marker expression in heat-shocked MDA-MB-468 cells, a potent enhancement of cell migration was still observed. The differences in the observed responses may reflect variations in epithelial plasticity between the two cell lines. Such variations have been observed previously in growth factor-induced EMT responses in differing cell line models, which have been attributed to general differences in epithelial plasticity and cell signalling pathway activation (Huber et al. 2005; Robson et al. 2006). Whether enhanced cell migration occurs universally in response to heat shock in other cancer and non-cancer cell lines awaits further study.
Immunohistological staining of thermally ablated liver metastases demonstrated an extensive reduction in E-cadherin staining as well as increased levels of Zeb1 in the surviving tumour fraction. Zeb1 is an E-cadherin transcriptional repressor, central for the promotion of EMT, metastasis and tumour progression (Spaderna et al. 2008). Although much research has focused on Zeb1 as a transcriptional repressor, increasing evidence has also indicated that Zeb1 can also transcriptionally activate target genes involved in EMT (Schmalhofer et al. 2009). Within this in vivo model, widespread downregulation of E-cadherin coupled with increased Zeb1 expression strongly demonstrated a shift towards a more mesenchymal phenotype within the surviving tumour cell fraction following thermal ablation. This finding suggests that areas of the tumour that do not reach sufficient temperatures to induce coagulative necrosis, undergo an EMT, highlighting the concept that administration of proteotoxic stress that is insufficient to cause cancer cell death may provide adverse effects through the induction of a more aggressive tumour phenotype. This study examined an acute cellular response (24 h) following thermal ablation, whether the EMT phenotype observed in this study persists over time remains to be delineated. However, Oliveira-Filho and colleagues have previously demonstrated that hyperthermic treatment of murine B16-F10 melanoma cell lines, although having a short-term negative impact upon cell viability and metastatic lung colonisation in mice, cells that were allowed to recover and injected into mice 13 days following heat shock treatment displayed an enhanced metastatic propensity (Oliveira-Filho et al. 1997). This points to a somewhat prolonged enhancement of metastatic phenotype induced by a single heat treatment.
In response to heat stress, a global reduction in RNA and protein synthesis is observed, as well as a loss in the functionality of many heat-sensitive proteins. In contrast, HSF1 maintains its functionality and becomes hyperactivated enabling it to transactivate the expression of HSP genes as well as potentially many other non-HSP genes. We therefore hypothesised that HSF1 may be a transcriptional mediator of cell migration and/or EMT in response to heat stress. Increased expression levels of HSF1 transcriptional targets HSP47 and HSPB1 have been associated with the mesenchymal phenotype (Wei et al. 2011; Zeisberg and Neilson 2009). In addition, O’Callaghan and Sherman demonstrated that immortalised hsf1−/− murine embryonic fibroblasts had a reduced capacity for migration (O’Callaghan-Sunol and Sherman 2006). However, in the current study, HSF1 knockdown in the A549 cell line did not inhibit basal levels of chemotactic cell migration, more surprisingly however, was the fact that knockdown of HSF1, although sufficient to prevent increased HSP expression induced by heat stress, was not able to inhibit the enhanced migration. This result indicates that heat stress illicits alterations to cancer cell biology features that are both dependent and independent of HSF1.
Cellular effects brought about by heat shock can be broad and non-specific, for example, heat shock has been shown to increase ROS levels within cells which in turn can activate a wide range of signalling pathways (Hildebrandt et al. 2002; Hsu et al. 2011; Jozwiak and Leyko 1992). Consistent with this, heat shock has been shown to activate numerous cell signalling pathways associated with migration and EMT, including c-Src, PI-3-kinase, the MAPK pathway and the EGFR pathway (Wolf et al. 2011; Lin et al. 1997; Nadeau and Landry 2007; Dubois and Bensaude 1993). Whether heat stress-induced activation of these pathways leads to increased cell migration and the activation of EMT associated transcription factors still need to be determined.
In addition to the HSR, another major stress pathway within cells is that of the unfolded protein response (UPR). Although these two responses are compartmentally separated, they share common elements and features, and it is known that upon heat shock, elements of the UPR are activated (Heldens et al. 2011). Therefore, the UPR may be an alternative stress pathway that may activate cellular migration and EMT programmes within the cancer cell. Consistent with such a role, it has been shown that mild activation of the UPR by ER stress induced by tunicamycin, thapsigargin or the overexpression of mutant surfactant protein-C (SP-C) induces EMT and cell migration in a number of cell types, including that of the A549 cell line (Zhong et al. 2011). Whether crosstalk between the HSR and UPR occurs, eliciting increased cellular migration and the EMT requires further examination.
An element that is shared between the HSR and the UPR is the proteasome to which misfolded or mutant proteins from the cytosol and the ER are targeted (Heldens et al. 2011). Inhibition of the proteasome generates a high degree of proteotoxic stress and is known to induce both the HSR and the UPR (Fribley et al. 2004; Neznanov et al. 2011). Proteasome inhibitors, such as Velcade®, are currently being clinically evaluated in combination with conventional chemotherapy in a number of cancer types to determine therapeutic efficacy (Yang et al. 2009). The rationale behind this approach is to generate proteotoxic stress levels that exceed the buffering capacity of the cancer cell stress pathways, generating ‘stress overload’ and inducing cancer cell death. As tumour cells exhibit higher levels of sensitivity to proteasome inhibition than non-transformed cells, it is believed that this approach will also afford a degree of tumour cell killing selectivity (Neznanov et al. 2011; Workman and Davies 2011; Dou and Li 1999). However, one potential caveat indicated by our present study is that if cell killing is not fully achieved then the emergence and/or recurrence of more advanced cancer phenotypes may result (Neznanov et al. 2011; Workman and Davies 2011; Dou and Li 1999). In previous studies, proteasome inhibition using a number of compounds has been shown to be both inhibitory and inductive towards EMT (Baritaki et al. 2009; Mak et al. 2010; Saitoh et al. 2009). Changes in the cancer cell phenotype induced by proteasome inhibition are likely to be dependent on the dosage and duration of inhibition. As these studies, including the current study, have used various cell models, compounds and treatments, the full extent to which proteasome inhibition influences a migratory phenotype requires further characterisation.
As proteasome inhibition also activates the HSR, it has been postulated that inhibition of the HSR may lead to increased toxicity of proteasome inhibitors, indeed Zaarur et al. have demonstrated in vitro inhibition of the HSR by knockdown of HSF1 sensitises several cancer cell lines to proteasome inhibition by MG132 (Zaarur et al. 2006). This has led to combination strategies to inhibit both the proteasome and HSR simultaneously to enhance the efficacy of proteasome inhibitory compounds (Workman and Davies 2011; Zaarur et al. 2006). Although knockdown of HSF1 may increase the toxicity of proteasome inhibitors, we determined that it was not able to prevent MG132 stimulated increases in A549 chemomigration. Thus, our findings indicate that inhibition of HSF1 in isolation would not be sufficient in preventing the recurrence of more advanced cancer phenotypes if the treatment proved inefficient at tumour cell killing.
In adult tissues, EMT has been shown to be important in wound healing, tissue fibrosis and cancer. These contexts are associated with high levels of cellular stress. Previous studies have demonstrated that EMT promotes resistance to apoptosis and chemotherapeutics and as such has led to the process being recognised as a survival mechanism of cancer cells (Tiwari et al. 2012; Li et al. 2009; Robson et al. 2006). This study has identified for the first time that heat stress can induce enhanced migratory capacities and the EMT in cancer cells independent of HSF1 signalling, and adds to the many studies that have provided evidence for EMT as a cellular response to sub-lethal proteotoxic stress. The enhanced malignant properties of recurrent tumour cells is often attributed to selection of a sub-population that possess enhanced survival and aggressive properties (Blagosklonny 2005b); however, the findings from this study suggest that the additional proteotoxic stress endured by these surviving cells may further potentiate their malignant phenotype.
Acknowledgments
The authors would like to acknowledge the staff at Monash Micro Imaging and Irene Hatzinisiriou (Monash University) for support in microscopy; staff at Flowcore (Monash University) for FACS support; Brendan Wilding (Monash University) for assistance with software analysis and Ashleigh Unsworth (Monash University) for critical reading of the manuscript. This work was supported by Cancer Council Victoria grant-in-aid no. 545969, National Health and Medical Research Council of Australia R Douglas Wright fellowship no. 395525 (JTP), Australian Postgraduate Award (BJL).
References
- Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of snail repression and RKIP induction. Oncogene. 2009;28(40):3573–3585. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Carcinogenesis, cancer therapy and chemoprevention. Cell Death Differ. 2005;12:592–602. doi: 10.1038/sj.cdd.4401610. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Why therapeutic response may not prolong the life of a cancer patient. Cell Cycle. 2005;4(12):1693–1698. doi: 10.4161/cc.4.12.2259. [DOI] [PubMed] [Google Scholar]
- Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zmara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29(12):2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- Chakraborty PK, Scharner B, Jurasovic J, Messner B, Bernhard D, Thevenod F. Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicol Lett. 2010;198(1):69–76. doi: 10.1016/j.toxlet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. The Ann Rev Cell Dev Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Dai S, Cao J. Proteotoxic stress of cancer: implication of the heat-shock response in oncogenesis. J Cell Physiol. 2012;227(8):2982–2987. doi: 10.1002/jcp.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updates. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- Dubois M, Bensaude O. MAP kinase activation during heat shock in quiescent and exponentially growing mammalian cells. FEBS Lett. 1993;324(2):191–195. doi: 10.1016/0014-5793(93)81391-C. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifis T, Malcontenti-Wilson C, Amijoyo J, Anggono B, Muralidharan V, Nikfarjam M, Christophi C. Changes in growth factor levels after thermal ablation in a murine model of colorectal liver metastases. HPB: Offic J Intl Hepatol Pancreatol Biliary Assoc. 2011;13(4):246–255. doi: 10.1111/j.1477-2574.2010.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34(1):19–31. doi: 10.1111/j.1530-0277.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24(22):9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MP, Cavalheiro JR, Fernandes MH. Acute and long-term effects of hyperthermia in b16-f10 melanoma cells. PLoS One. 2012;7(4):e35489. doi: 10.1371/journal.pone.0035489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida N, Fujimoto M, Tan K, Prakasam R, Shinkawa T, Li L, Ichikawa H, Takii R, Nakai A. Heat shock factor 1 ameliorates proteotoxicity in cooperation with the transcription factor NFAT. EMBO J. 2010;29(20):3459–3469. doi: 10.1038/emboj.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldens L, Hensen SM, Onnekink C, Genesen ST, Dirks RP, Lubsen NH. An atypical unfolded protein response in heat shocked cells. PLoS One. 2011;6(8):e23512. doi: 10.1371/journal.pone.0023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol/Hematol. 2002;43:33–56. doi: 10.1016/S1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Yu H, Lin H, Wu K, Yang R, Kuo P. Heat shock induces apoptosis through reactive oxygen species involving mitochondrial and death receptor pathways in corneal cells. Exp Eye Res. 2011;93(4):405–412. doi: 10.1016/j.exer.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Huff CA, Matsui W, Smith BD, Jones RJ. The paradox of response and survival in cancer therapeutics. Blood. 2006;107(2):431–434. doi: 10.1182/blood-2005-06-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianaro A, Ialenti A, Maffia P, Pisano B, Rosa M. HSF1/hsp72 pathway as an endogenous anti-inflammatory system. FEBS Lett. 2001;499(3):239–244. doi: 10.1016/S0014-5793(01)02569-8. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;19:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Jozwiak Z, Leyko W. Role of the membrane components in thermal injury of cells and development of thermotolerance. Int J Radiat Biol. 1992;62(6):743–756. doi: 10.1080/09553009214552701. [DOI] [PubMed] [Google Scholar]
- Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816(2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Kouspou MM, Price JT. Analysis of cellular migration using a two-chamber methodology. Methods Mol Biol. 2011;787:303–317. doi: 10.1007/978-1-61779-295-3_23. [DOI] [PubMed] [Google Scholar]
- Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, Chen ZQ, Liu XP, Xu ZD. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15(8):2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- Lin RZ, Hu Z-W, Chin JH, Hoffman BB. Heat shock activates c-Src tyrosine kinases and phosphatidylinositol 3-kinase in NIH3T3 fibroblasts. J Biol Chem. 1997;272(49):31196–31202. doi: 10.1074/jbc.272.49.31196. [DOI] [PubMed] [Google Scholar]
- Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity TK, Henry MM, Tulapurkar ME, Shah NG, Hasday JD, Singh IS. Distinct, gene-specific effect of heat shock on heat shock factor-1 recruitment and gene expression of CXC chemokine genes. Cytokine. 2011;54(1):61–67. doi: 10.1016/j.cyto.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17(4):319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan RD, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273(13):7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Nadeau SI, Landry J. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv Exp Med Biol. 2007;594:100–113. doi: 10.1007/978-0-387-39975-1_10. [DOI] [PubMed] [Google Scholar]
- Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV. Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget. 2011;2(3):209–221. doi: 10.18632/oncotarget.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikfarjam M, Muralidharan V, Su K, Malcontenti-Wilson C, Christophi C. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperth. 2005;21(4):319–332. doi: 10.1080/02656730500133736. [DOI] [PubMed] [Google Scholar]
- O’Callaghan-Sunol C, Sherman MY. Heat shock transcription factor (HSF1) plays a critical role in cell migration via maintaining MAP kinase signaling. Cell Cycle. 2006;5(13):1431–1437. doi: 10.4161/cc.5.13.2915. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho RS, Bevilacqua RG, Chammas R. Hyperthermia increases the metastatic potential of murine melanoma. Braz J Med Biol Res. 1997;30:941–945. doi: 10.1590/S0100-879X1997000800005. [DOI] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page TJ, Sikder D, Yang L, Pluta L, Wolfinger RD, Kodadek T, Thomas RS. Genome-wide analysis of human HSF1 signaling reveals a transcriptional program linked to cellular adaptation and survival. Mol Biosyst. 2006;2(12):627–639. doi: 10.1039/b606129j. [DOI] [PubMed] [Google Scholar]
- Price JT, Quinn JM, Sims NA, Vieusseux J, Waldeck K, Docherty SE, Myers D, Nakamura A, Waltham MC, Gillespie MT, Thompson EW. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 2005;65(11):4929–4938. doi: 10.1158/0008-5472.CAN-04-4458. [DOI] [PubMed] [Google Scholar]
- Robson EJD, Khaled WT, Abell K, Watson CJ. Epithelial-to-mesenchymal transition confers resistance to apoptosis in three murine mammary epithelial cell lines. Differentiation. 2006;74(5):254–264. doi: 10.1111/j.1432-0436.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann N Y Acad Sci. 2005;1066:222–242. doi: 10.1196/annals.1363.009. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Shirakihara T, Miyazono K. Regulation of the stability of cell surface E-cadherin by the proteasome. Biochem Biophys Res Commun. 2009;381(4):560–565. doi: 10.1016/j.bbrc.2009.02.098. [DOI] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exper Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. PNAS. 2011;108(45):18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28(1–2):151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, He JR, Hasday JD. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39(2):235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, Kirchner T, Behrens J, Brabletz T. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68(2):537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- Tamminen JA, Myllärniemi M, Hyytiäinen M, Keski-Oja J, Koli K (2012) Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling. J Cell Biochem:n/a-n/a. doi:10.1002/jcb.24094 [DOI] [PubMed]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tiwari N, Gheldof A, Tatari M, Christofori G (2012) EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. doi:10.1016/j.semcancer.2012.02.013 [DOI] [PubMed]
- Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell. 2004;15(3):1254–1261. doi: 10.1091/mbc.E03-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaboa NE, Galan A, Troyano A, Blas E, Aller P. Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1) J Biol Chem. 2000;275(32):24970–24976. doi: 10.1074/jbc.M909136199. [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15(3):138–145. doi: 10.1016/j.tcb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Wei L, Liu TT, Wang HH, Hong HM, Yu AL, Feng HP, Chang WW. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res: BCR. 2011;13(5):R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin Ther Targets. 2009;13(4):469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- Wolf F, Li W, Li F, Li C. Non-invasive, quantitiative monitoring of hyperthermia-induced EGFR activation in xenograft tumours. Int J Hyperth. 2011;27(5):427–434. doi: 10.3109/02656736.2011.566593. [DOI] [PubMed] [Google Scholar]
- Workman P, Davies FE. A stressful life (or death): combinatorial proteotoxic approaches to cancer0selective therapeutic vulnerability. Oncotarget. 2011;2(4):277–280. doi: 10.18632/oncotarget.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18(21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Huang S. Regulation of cancer metastasis by stress pathways. Clin Exp Metastasis. 2003;20:31–43. doi: 10.1023/A:1022590402748. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci. 2009;100(1):24–28. doi: 10.1111/j.1349-7006.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaarur N, Gabai VL, Porco JA, Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66(3):1783–1791. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, Kim KJ, duBois RM, Crandall ED, Beers MF, Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Molr Biol. 2011;45(3):498–509. doi: 10.1165/rcmb.2010-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]