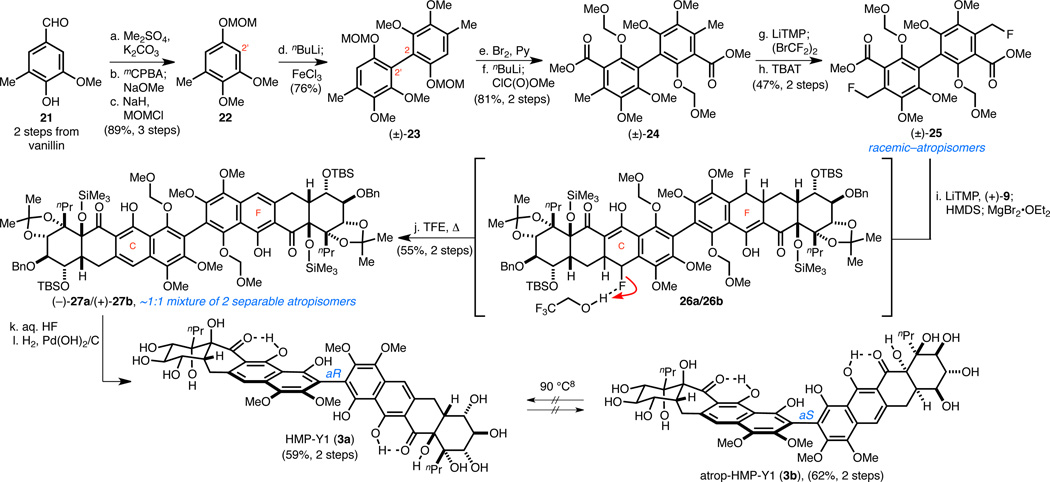
Scheme 5. Synthesis of HMP-Y1 (3a) and atrop-HMP-Y1 (3b) via a Benzyl Fluoride Michael–Claisen Reaction Sequence.
aConditions: (a) Me2SO4, K2CO3, Me2CO, 98%; (b) mCPBA, NaHCO3, CH2Cl2; then Na2CO3, MeOH; (c) NaH, MOMCl, DMF, 0 °C → RT, 91% (two steps); (d) nBuLi, TMEDA, THF, −78 → 0 °C; then FeCl3, 0 °C → RT, 76%; (e) Br2, Py, CH2Cl2, 0 °C, 91%; (f) nBuLi, THF, −78 °C; then ClC(O)OMe, −78 °C → RT, 89%; (g) LiTMP, THF, −78 °C; then (BrCF2)2, −78 °C, 62%; (h) TBAT, MeCN, 82 °C, 75%; (i) (+)-9, LiTMP, THF, −78 °C; then HMDS, −78 → −35 °C; then MgBr2•OEt2, −35 → 0 °C; (j) CF3CH2OH/H2O, NaHCO3, 80 °C, 55% (two steps); (k) aq. HF, MeCN/THF, 50 °C; (l) H2, Pd(OH)2/C, THF; for 3a, 59% (two steps); for 3b, 62% (two steps).