SECTION 1
A 47-year-old right-handed man, with no history of alcohol use, presented with episodic unsteadiness that began in his early 20s. During the episodes, which last several hours, he is unable to walk steadily and has poor control of his limbs. These attacks are often brought on by emotional stress and occurred 1 to 2 times per month into his 30s. There is no association with headache or head movement, no diplopia, tinnitus, or hearing loss. His earlier evaluation included a brain MRI and routine EEG, which were normal. Though his diagnosis was unknown, he was given a trial of acetazolamide at age 38, and became attack-free on the medication.
Questions for consideration:
What is the differential diagnosis for paroxysmal episodes of neurologic dysfunction based on the time course and age at onset?
How can medication responsiveness and examination findings be helpful?
GO TO SECTION 2
SECTION 2
In this patient, the likely etiologies for paroxysmal neurologic events are seizures, migraine, vestibular syndromes, and paroxysmal movement disorders. Pure ataxia as seizure semiology has not been described: cerebellar cortex is not known to be epileptogenic. Vertiginous partial seizures may be localized to the posterior part of superior temporal neocortex, are rare, and typically seen in conjunction with other temporal lobe localizing symptoms (auditory, olfactory). Postictal state of complex partial or generalized seizures can result in gait unsteadiness associated with fatigue and confusion and gradual improvement.
In migraine, paroxysmal neurologic symptoms may occur without headache. Classified as typical aura without headache, the aura must include visual or sensory symptoms, and last less than 1 hour.1 If migraines, the patient's episodes fit most closely with the aura of basilar-type migraine, which may include dysarthria, vertigo, diplopia, ataxia, tinnitus or hyperacusis, decreased level of consciousness, or bilateral paresthesias. However, as defined by the International Headache Society, this aura may last up to 1 hour and must be associated with migraine headache.1
Vestibular syndromes include Ménière disease, which is less likely given the lack of auditory symptoms, and benign recurrent vertigo, etiologically thought to be related to migraine.2
Of the paroxysmal movement disorders, the patient's symptoms best fit an episodic ataxia (EA). While many types have been described, the most common EA syndromes are type 1 (EA1) with episodes lasting seconds to minutes, often precipitated by startle or movement; and type 2 (EA2) with episodes lasting hours, commonly precipitated by emotional stress.3,4 Response to acetazolamide, which has limited usefulness in epilepsy due to tolerance, is common in EA2, but is only seen occasionally in EA1.
Several months prior to current evaluation, the patient was taken off acetazolamide after undergoing treatment for squamous cell cancer of the neck, due to concerns for dehydration. Two weeks after stopping the medication, his ataxic attacks recurred, occurring several times per week, lasting several hours, and now associated with slurred speech. He also developed severe pounding headaches during most attacks, without nausea, photophobia, or phonophobia.
His neurologic examination, performed between episodes, was normal, including absence of nystagmus, dysarthria, gait ataxia, or dysmetria. In most paroxysmal disorders in the differential and in some patients with EA, the interictal examination is normal. In EA2, patients may develop mild interictal ataxia, and different forms of nystagmus, including gaze-evoked nystagmus, rebound nystagmus, and spontaneous vertical nystagmus.4 In EA1, myokymia can be observed.
Question for consideration:
1. What other historical information is critical for diagnosis?
GO TO SECTION 3
SECTION 3
Many of the paroxysmal movement disorders have typical patterns of inheritance; both EA1 and EA2, caused by mutations in different genes, display an autosomal dominant pattern. The patient's mother has mild episodes of gait instability and dysarthria lasting hours, occurring several times per month, precipitated by anxiety or excitement. Her episodes started at age 11 without progressive nature. The patient's sister, age 41, has episodes of severe vertigo, nausea, and inability to walk that started at age 12. Ten years ago, acetazolamide was started and led to a dramatic reduction in attack frequency. She also has frequent headaches that meet criteria for migraine with visual aura. Sometimes her migraines trigger an episode of ataxia.
Questions for consideration:
How is the clinical diagnosis of episodic ataxia made?
What is the definitive diagnostic test for episodic ataxia type 2?
GO TO SECTION 4
SECTION 4
The diagnosis of EA2, consistent with the presentation in this patient, is most commonly made on clinical grounds based on 1) attacks of gait ataxia and nystagmus lasting hours, with possible associated vertigo, nausea, vomiting, dysarthria; 2) possible presence of interictal ataxia and nystagmus or progressive ataxia; 3) attacks provoked by exercise, emotional stress; 4) attacks reduced in frequency by acetazolamide; 5) absence of myokymia; 6) family history consistent with autosomal dominant inheritance; 7) onset before age 20.3,4 Other clinical features include associated headache, fluctuating generalized weakness, seizures, and dystonia.3
Definitive diagnosis of EA2 is made with genetic testing. Sequencing of CACNA1A gene (Athena Diagnostics) revealed a previously undescribed nonsense mutation at arginine 1346 (i.e., R1346X, corresponding to nucleotide C4036T). The patient was restarted on acetazolamide with titration up to 750 mg/day. He has had only 1 episode of ataxia over the next year and his headache has not recurred.
DISCUSSION
The CACNA1A gene codes for the main transmembrane pore-forming and voltage-sensing subunit of the P/Q-type voltage-gated calcium channel (Cav2.1). Mutations in CACNA1A cause a spectrum of disorders with overlapping clinical features, termed CACNA1A channelopathies. Clinical syndromes include EA2 (nonsense >missense mutations), familial hemiplegic migraine 1 (FHM1) (missense type), spinocerebellar ataxia type 6 (polymorphic CAG repeat expansions), and epilepsy.5–7
Sequencing of the CACNA1A gene in our patient revealed a novel nonsense mutation, R1346X, located in the fourth transmembrane segment of the homologous domain III of the channel pore-forming unit. A missense mutation at the same position, R1346Q, has been described in a family with FHM1 and features of EA2.4 Cav2.1 channels are involved in the release of neurotransmitter at multiple types of synapses, with a vital role in cerebellar Purkinje cells. Truncating mutations in Cav2.1 channels lead to dysfunctional neurotransmission, as supported by a reduction in current density from mutated Cav2.1 in ataxic mouse models.4 Gain-of-function effects with increased calcium current density are found in FHM1 mouse models, resulting in increased calcium-uptake related glutamate release and a lower threshold for cortical spreading depression, thought to be the pathophysiologic mechanism underlying migraine with aura.5 In humans, nearly identical mutations may result in both FHM1 and EA2 phenotypes,4 suggesting a more complex relationship between Cav2.1 activity and clinical phenotype.
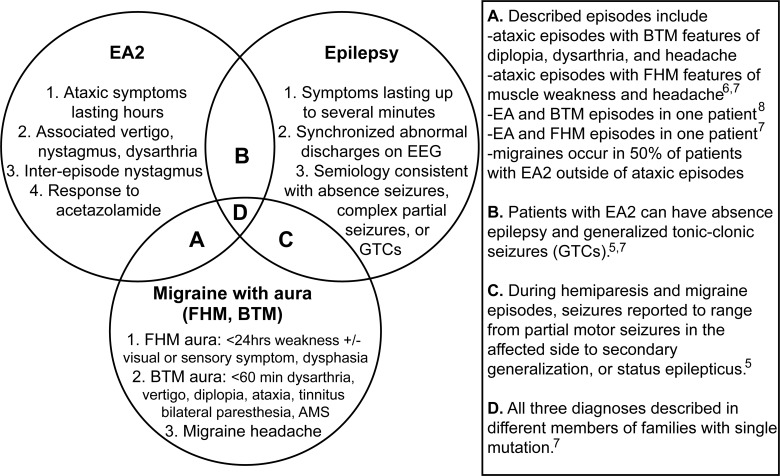
Several paroxysmal disorders, both CACNA1A-related and -unrelated, share considerable symptom overlap. Conversely, identical CACNA1A mutations may produce phenotypes that meet criteria for 2 or 3 disease categories, either in individual patients or in different family members.3,5–7 Furthermore, a single stereotyped episode can have features of multiple disorders. The figure summarizes the overlapping and unique symptoms in paroxysmal disorders that have been associated with CACNA1A mutations, including EA2, migraine with aura, and epilepsy.
Figure. Phenotypic overlap of paroxysmal disorders that have been associated with CACNA1A mutations.
Key diagnostic criteria and overlapping features are summarized for episodic ataxia type 2 (EA2), migraine with aura including familial hemiplegic migraine 1 (FMH1) and basilar-type migraine (BTM), and epilepsy. One individual may have different types of episodes meeting criteria for multiple disorders, or because of many shared symptoms, single stereotyped episodes may meet most criteria for multiple disorders. Overlap between diagnostic categories is indicated on the Venn diagram with a letter, with specific details and references to the described cases listed in the adjacent table.
About half of patients with EA2 (such as this patient's sister) have headaches that meet formal criteria for migraine.4 FHM1 diagnostic criteria include an aura of motor weakness with visual, sensory, or speech symptoms lasting less than 24 hours, in addition to migraine headache. Up to 50% of patients with FHM1 will have interepisode progressive cerebellar symptoms.1 Migraines with muscle weakness have been reported in association with cerebellar dysfunction, meeting criteria for both EA2 and FHM1.6,7 Basilar-type migraine (BTM) has the greatest overlap in symptomatology with EA2. Its underlying genetic causes are under investigation. In one case report, 2 different types of episodes were diagnosed as BTM and EA2 in a single patient with a CACNA1A truncating mutation, with acetazolamide reducing the frequency of both episodes.8 Epilepsy and nonepileptiform EEG abnormalities6 in families with CACNA1A mutations have also been studied. Absence, complex partial, and generalized tonic-clonic seizures have been described in the setting of severe hemiplegic migraine attacks,5 or independent of attacks in patients with FHM1 or EA2.3,5,7
Appreciation of the spectrum of disorders associated with CACNA1A mutation may suggest additional treatment options for EA2. The standard of care, acetazolamide, reduces frequency and severity of attacks in 71% of patients,3 but has been reported to fail over time.9 An alternative prophylactic agent is 4-aminopyridine, a potassium channel blocker.9 Valproic acid is the only other agent that has been reported to have benefit in EA2.10 Agents with both antiepileptic and migraine prophylaxis properties are potential alternatives for investigation. For example, topiramate and zonisamide possess several antiepileptic channel effects as well as carbonic anhydrase inhibitory activity, similar to acetazolamide.
In our patient, the clinical and genetic diagnosis of EA2 helps guide the selection of first-line therapy and suggests potential future treatment options. In his sister, identification of comorbid conditions should lead to treatment of both, reducing the likelihood of one triggering another. Our case highlights the clinical heterogeneity and overlap among CACNA1A channelopathies and ataxia-related paroxysmal disorders more broadly, which can aid in timely diagnosis and appropriate management of these conditions.
AUTHOR CONTRIBUTIONS
Dr. Yugrakh developed the study concept, participated in analysis and interpretation of data, and drafted and revised the manuscript. Dr. Levy developed the study concept, participated in analysis and interpretation of data, and revised the manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Headache Classification Subcommittee of International Headache Society The International Classification of Headache Disorders, 2nd ed. Cephalalgia 2004; 24 (suppl 1): 9– 160 [DOI] [PubMed] [Google Scholar]
- 2. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms: identifying clinical features that predict “vestibular migraine.” Headache 2011; 51: 1393– 1397 [DOI] [PubMed] [Google Scholar]
- 3. Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology 2004; 62: 17– 22 [DOI] [PubMed] [Google Scholar]
- 4. Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW. Primary episodic ataxias: diagnosis, pathogenesis, and treatment. Brain 2007; 130: 2484– 2493 [DOI] [PubMed] [Google Scholar]
- 5. Haan J, Terwindt GM, van den Maagdenberg AMJM, Stam AJ, Ferrari MD. A review of the genetic relation between migraine and epilepsy. Cephalalgia 2008; 28: 105– 113 [DOI] [PubMed] [Google Scholar]
- 6. Romaniello R, Zucca C, Tonelli A, et al. A wide spectrum of clinical, neurophysiological and neuroradiological abnormalities in a family with a novel CACNA1A mutation. J Neurol Neurosurg Psychiatry 2010; 81: 840– 843 [DOI] [PubMed] [Google Scholar]
- 7. Jung J, Testard H, Tournier-Lasserve E, et al. Phenotypic variability of episodic ataxia type 2 mutations: a family study. Eur Neurol 2010; 64: 114– 116 [DOI] [PubMed] [Google Scholar]
- 8. Robbins MS, Lipton RB, Laureta EC, Grosberg BM. CACNA1A nonsense mutation is associated with basilar-type migraine and episodic ataxia type 2. Headache 2009; 49: 1042– 1046 [DOI] [PubMed] [Google Scholar]
- 9. Strupp M, Kalla R, Claassen J, et al. A randomized trial of 4-aminopyrindine in EA2 and related familial episodic ataxias. Neurology 2011; 77: 269– 275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scoggan KA, Friedman JH, Bulman DE. CACNA1A mutation in EA-2 patient responsive to acetazolamide and valproic acid. Can J Neurol Sci 2006; 33: 68– 72 [DOI] [PubMed] [Google Scholar]