Abstract
AIM: To compare the volumetric-modulated arc therapy (VMAT) plans with conventional sliding window intensity-modulated radiotherapy (c-IMRT) plans in esophageal cancer (EC).
METHODS: Twenty patients with EC were selected, including 5 cases located in the cervical, the upper, the middle and the lower thorax, respectively. Five plans were generated with the eclipse planning system: three using c-IMRT with 5 fields (5F), 7 fields (7F) and 9 fields (9F), and two using VMAT with a single arc (1A) and double arcs (2A). The treatment plans were designed to deliver a dose of 60 Gy to the planning target volume (PTV) with the same constrains in a 2.0 Gy daily fraction, 5 d a week. Plans were normalized to 95% of the PTV that received 100% of the prescribed dose. We examined the dose-volume histogram parameters of PTV and the organs at risk (OAR) such as lungs, spinal cord and heart. Monitor units (MU) and normal tissue complication probability (NTCP) of OAR were also reported.
RESULTS: Both c-IMRT and VMAT plans resulted in abundant dose coverage of PTV for EC of different locations. The dose conformity to PTV was improved as the number of field in c-IMRT or rotating arc in VMAT was increased. The doses to PTV and OAR in VMAT plans were not statistically different in comparison with c-IMRT plans, with the following exceptions: in cervical and upper thoracic EC, the conformity index (CI) was higher in VMAT (1A 0.78 and 2A 0.8) than in c-IMRT (5F 0.62, 7F 0.66 and 9F 0.73) and homogeneity was slightly better in c-IMRT (7F 1.09 and 9F 1.07) than in VMAT (1A 1.1 and 2A 1.09). Lung V30 was lower in VMAT (1A 12.52 and 2A 12.29) than in c-IMRT (7F 14.35 and 9F 14.81). The humeral head doses were significantly increased in VMAT as against c-IMRT. In the middle and lower thoracic EC, CI in VMAT (1A 0.76 and 2A 0.74) was higher than in c-IMRT (5F 0.63 Gy and 7F 0.67 Gy), and homogeneity was almost similar between VMAT and c-IMRT. V20 (2A 21.49 Gy vs 7F 24.59 Gy and 9F 24.16 Gy) and V30 (2A 9.73 Gy vs 5F 12.61 Gy, 7F 11.5 Gy and 9F 11.37 Gy) of lungs in VMAT were lower than in c-IMRT, but low doses to lungs (V5 and V10) were increased. V30 (1A 48.12 Gy vs 5F 59.2 Gy, 7F 58.59 Gy and 9F 57.2 Gy), V40 and V50 of heart in VMAT was lower than in c-IMRT. MUs in VMAT plans were significantly reduced in comparison with c-IMRT, maximum doses to the spinal cord and mean doses of lungs were similar between the two techniques. NTCP of spinal cord was 0 for all cases. NTCP of lungs and heart in VMAT were lower than in c-IMRT. The advantage of VMAT plan was enhanced by doubling the arc.
CONCLUSION: Compared with c-IMRT, VMAT, especially the 2A, slightly improves the OAR dose sparing, such as lungs and heart, and reduces NTCP and MU with a better PTV coverage.
Keywords: Esophageal cancer, Treatment planning, Intensity modulated radiotherapy, Volumetric modulated arc radiotherapy, Normal tissue complication probability
INTRODUCTION
Esophageal cancer (EC) is one of the most common malignancies in the world. It was estimated that there were 16 470 newly diagnosed cases of EC, and 14 280 cases of death in America in 2008[1]. Squamous cell carcinoma is commonly seen in China, whereas adenocarcinoma is common in Europe and America. Radiotherapy is a major treatment method for EC because more than 60% of the patients are often diagnosed at locally advanced stages which could not be totally resected. Innovative technologies in radiation delivery such as intensity-modulated radiotherapy (IMRT) offer the potential for improved tumor coverage, while reducing the doses delivered to the surrounding normal tissues. Clinical studies have yielded good dosimetry and patient outcome by IMRT[2-6]. There are different IMRT delivery approaches, including “step and shoot”, “sliding window” modes and the rotational technique. Volumetric-modulated arc therapy (VMAT), the novel form of IMRT that was first proposed by Yu in 1995[7], allowed for intensity-modulated radiation delivery during gantry rotation with dynamic multi-leaf collimator (MLC) motion, variable dose rates (DR) and gantry speed modulation. VMAT had already been investigated for prostate cancer, small brain tumors and cervix uteri cancer[8-10]. These studies have generally shown that VMAT is able to produce similar or better dose distributions, while achieving a reduction in treatment time and a reduction in monitor units (MU).
We performed a planning study to compare VMAT with conventional sliding window intensity-IMRT (c-IMRT) in EC of all locations and in dose distributions to planning target volume (PTV) and organs at risk (OAR). We also investigated the difference of normal tissue complication probability (NTCP) between the two techniques.
MATERIALS AND METHODS
Patients
Twenty EC patients treated with c-IMRT previously in our department were selected for this study, involving 5 cases of EC located in the cervical, the upper, the middle and the lower thorax, respectively. Five patients were staged II, 10 were III and 5 were IV according to the American Joint Committee (AJCC) on Cancer 2006 Guidelines. Details are shown in Table 1.
Table 1.
Characteristics of patients (n = 20)
| Variables | n |
| Gender | |
| Male | 16 |
| Female | 4 |
| Age range (yr) | 45-82 |
| Stage1 | |
| II | 5 |
| III | 10 |
| IV | 5 |
| Histology | |
| Squamous carcinoma | 18 |
| Adenocarcinoma | 2 |
According to the American Joint Committee on Cancer 2006 Guidelines.
Target volume and organ at risk delineation
All patients were immobilized in a supine position and computed tomography scanned using a helical scanner (Siemens Somatom, Sensation Open Computed Tomography) with 1.25 mm thick slices over the neck and the entire thorax. The clinical target volume, including the esophageal tumor, with a margin for microscopic tumor extension, and the adjacent regional lymph nodes[11,12], was expanded with a 5-mm margin to create PTV. OAR, such as spinal cord, heart and lung, was outlined on each image. Details of the delineation of these volumes were recently described[13].
Planning techniques and objectives
All the treatment plans were designed to deliver 60 Gy to the PTV in 30 fractions using the Eclipse treatment planning system (Version 8.9, Varian Medical Systems, Palo Alto, CA), with 6 MV photon beam from a Varian IX (RapidArc) equipped with a Millennium MLC with 120 leaves. The Anisotropic Analytical Algorithm (Version 8.9) photon dose calculation algorithm and dose calculation grid of 2.5 mm were used for both c-IMRT and VMAT. When necessary, field size was minimized to 15.3 cm in the X direction. This dimension corresponded of the maximal displacement of a leaf in a MLC bank. By doing so, all the leave positions were possible during the optimization process increasing the degree of modulation even if in a beam eye view, a part of the volume was excluded of the beam at each gantry position. Globally rotational delivery permitted to irradiate all the volume of the PTV during rotation. All plans aimed to achieve a minimum dose larger than 95% and a maximum dose lower than 107% of the prescribed dose, and no 2-cc region (either in or outside of PTV) may receive > 110% of the dose. With regard to the OAR, the primary objectives were defined as follows: spinal cord: Dmax < 45 Gy and lungs: V20 < 30%. The secondary objectives were: mean doses of lungs (MLD) < 15 Gy and heart: V40 ≤ 50%, V50 ≤ 40%. As a result of tumor coverage requirements, a waiver can be applied on these dose constraints.
VMAT plans
The VMAT plans using full arcs sharing the same isocenter, in which 1A consisting of a single 360° rotation and 2A consisting of two coplanar arcs of 360° with opposite rotation (clock-wise or counter clock-wise), were optimized selecting a maximum DR of 600 MU/min. For 1A, starting at a gantry angle of 179° and rotating counter clockwise at 360° to stop at a gantry angle of 181°, field size and collimator rotation were determined by the automatic tool from Eclipse to encompass the PTV. And 2A, consisting of two coplanar arcs of 360°, was optimized simultaneously with opposite rotation. Since each individual arc is limited to a sequence of 177 control points, the application of two coplanar arcs that increase the modulation factor during optimization, could allow the optimizer to achieve a higher target homogeneity and lower OARs involvement at the same time. For the second arc, the collimator was rotated 5° extra to reduce overlapping tongue and groove effects with the first arc. Details about VMAT optimization process have been published elsewhere[14].
c-IMRT plans
The c-IMRT plans were optimized with a fixed DR of 400 MU/min. The MLC leaf sequences were generated using the dynamic sliding window IMRT delivery technique. Plans were individually optimized using five (5F), seven (7F) and nine (9F) coplanar fields. Beam geometry consisted of each treatment field with the following gantry angles: 0°/50°/153°/204°/310° (5F), 20°/60°/150°/180°/210°/300°/340° (7F), and 0°/35°/70°/130°/160°/200°/230°/290°/325° (9F).
Once the treatment planning was completed, the plan was normalized to cover 95% of the PTV with 100% of the prescribed dose. In the present study, we tried to modify constraints and priority factors in the c-IMRT and VMAT plans to improve the results. These parameters were modified in function of dose-volume histogram (DVH) results for each patient.
Evaluation tools
Analysis was performed on DVH computing several standard parameters[15], Dx was the specific dose computed for a fraction of a target or an organ volume, and Vx was the volume irradiated above a designated dose. For PTV, the mean dose (Dmean) was analyzed, and the conformity of dose distribution was assessed by means of conformity index (CI) which was defined as the ratio between the volume receiving at least 95% of 60 Gy and the volume of the PTV. Higher values of CI represented a better PTV conformity. CI = (VT95%/VT) × (VT95%/V95%)[16].
The homogeneity index (HI) of the PTV was computed as D5%-D95% (difference between the dose covering 5% and 95% of the PTV). Lower values of HI represented a more homogenous PTV dose distribution[17].
DVH parameters for OARs (spinal cord, lungs and heart) were calculated and compared. A set of Vx values, Dmean, Dmax and MU was therefore reported.
Radiobiological comparison was analyzed by the NTCP. The risk of developing acute complications and other late complications was assessed using the Lyman-Kutcher-Burman model[18]. The parameters for NTCP calculations (volume effect, slope, and tolerance doses) were taken from Burman et al[19] and are shown in Table 2.
Table 2.
Parameters used in normal tissue complication probability
| Organ | Size factor (n) | Slope (m) | TD5/5 (Gy) | TD50/5 (Gy) | End point |
| Lung | 0.87 | 0.18 | 17.5 | 24.5 | Pneumonitis |
| Heart | 0.35 | 0.10 | 40 | 48 | Pericarditis |
| Spinal cord | 0.05 | 0.175 | 47 | 66.5 | Myelitis/necrosis |
TD5/5: Tolerance dose leading to 5% complication rates at 5 years; TD50/5: Tolerance dose leading to 50% complication rates at 5 years.
Statistical analysis
The Wilcoxon matched-pair signed-rank test was used to compare the results between the VMAT and IMRT plans. Difference was considered statistically significant at P < 0.05. All statistical tests were two-sided, and all statistical analyses were done using the SPSS software, Version 11.0 (Chicago, IL).
RESULTS
Target coverage, conformity and dose homogeneity
Clinically acceptable plans of VMAT and c-IMRT were completed by all the 20 patients. The dosimetric results of each position for PTV are listed in Table 3. The results were analogous in the cervical and upper EC, for which PTV was T-shaped from a posteroanterior view, while PTV was I-shaped in middle and lower EC. As the numbers of field in c-IMRT or arc in VMAT were increased, the conformity and homogeneity were improved.
Table 3.
Dosimetric results for planning target volume and monitor units
| Variable | IMRT-5F | IMRT-7F | IMRT-9F | VMAT-1A | VMAT-2A | P < 0.05 |
| Dmean | ||||||
| Cervical | 63.68 ± 0.37 | 63.07 ± 0.36 | 62.55 ± 0.39 | 63.97 ± 0.08 | 63.63 ± 0.49 | 2A vs 7F and 9F |
| Upper | 63.30 ± 0.66 | 62.74 ± 0.49 | 62.38 ± 0.34 | 63.90 ± 0.45 | 63.43 ± 0.63 | 1A, 2A vs 7F and 9F |
| Middle | 64.12 ± 1.03 | 64.05 ± 1.27 | 63.88 ± 1.27 | 64.83 ± 1.06 | 64.21 ± 0.59 | 1A vs 5F and 9F |
| Lower | 63.14 ± 0.90 | 63.20 ± 1.09 | 62.98 ± 0.87 | 64.17 ± 1.26 | 63.98 ± 1.36 | 1A vs 5F, 7F and 9F |
| HI | ||||||
| Cervical | 1.10 ± 0.01 | 1.09 ± 0.01 | 1.07 ± 0.01 | 1.11 ± 0.00 | 1.10 ± 0.01 | 1A, 2A vs 7F and 9F |
| Upper | 1.09 ± 0.02 | 1.08 ± 0.01 | 1.07 ± 0.01 | 1.10 ± 0.01 | 1.09 ± 0.02 | 1A vs 7F and 9F; 2A vs 9F |
| Middle | 1.11 ± 0.02 | 1.11 ± 0.03 | 1.11 ± 0.03 | 1.12 ± 0.02 | 1.11 ± 0.01 | 1A vs 9F |
| Lower | 1.09 ± 0.02 | 1.09 ± 0.03 | 1.09 ± 0.02 | 1.11 ± 0.04 | 1.11 ± 0.03 | 1A vs 9F |
| CI | ||||||
| Cervical | 0.63 ± 0.03 | 0.66 ± 0.02 | 0.74 ± 0.04 | 0.78 ± 0.03 | 0.80 ± 0.03 | 2A vs 5F, 7F, 9F and 1A |
| Upper | 0.62 ± 0.04 | 0.66 ± 0.03 | 0.73 ± 0.02 | 0.79 ± 0.03 | 0.80 ± 0.02 | 1A, 2A vs 5F, 7F and 9F |
| Middle | 0.62 ± 0.09 | 0.67 ± 0.08 | 0.71 ± 0.10 | 0.76 ± 0.05 | 0.74 ± 0.08 | 1A, 2A vs 5F and 7F |
| Lower | 0.64 ± 0.05 | 0.67 ± 0.05 | 0.71 ± 0.05 | 0.76 ± 0.05 | 0.77 ± 0.04 | 1A, 2A vs 5F and 7F |
| MU | ||||||
| Cervical | 1088 (921-1157) | 1261 (1094-1393) | 1236 (1004-1413) | 610 (546 -665) | 525 (452-590) | 2A vs 5F, 7F, 9F and 1A |
| Upper | 1110 (841-1244) | 1251 (950-1377) | 1334 (1040-1592) | 679 (538-825) | 682 (475-1004) | 1A, 2A vs 5F, 7F and 9F |
| Middle | 831 (707-980) | 903 (808-1086) | 999 (858-1219) | 418 (347-459) | 431 (376-503) | 1A, 2A vs 5F, 7F and 9F |
| Lower | 826 (721-966) | 923 (720-1234) | 1086 (958-1375) | 440 (387-540) | 419 (347-531) | 1A , 2A vs 5F, 7F and 9F |
IMRT: Intensity modulated radiotherapy; VMAT: Volumetric modulated arc therapy; F: Coplanar field; A: Arc; HI: Homogeneity index; CI: Conformity index; Dmean: Mean dose; MU: Monitor units.
For Dmean of PTV, VMAT (1A and 2A) yielded higher values than IMRT (5F, 7F and 9F). There was significant difference between VMAT and c-IMRT (7F and 9F) in cervical and upper thoracic EC and c-IMRT (5F and 7F) in the middle and lower thoracic EC, and only 1A achieved a higher Dmean as compared with IMRT (P < 0.05).
VMAT had a better CI than c-IMRT. Statistically significant difference was seen between VMAT and c-IMRT (5F, 7F and 9F) in cervical and upper thoracic EC, but between VMAT and c-IMRT (5F and 7F) in middle and lower thoracic EC (P < 0.05). Especially in cervical cases, 2A showed the best CI (P < 0.05), but there was no significant difference between 1A and 2A in thoracic cases.
Homogeneity was slightly better in c-IMRT than in VMAT. In cervical and upper thoracic EC, HI of 2A and 5F was equivalent, and 7F or 9F showed a significant trend for better results compared with VMAT (P < 0.05). In the middle and lower thoracic EC, the trend was not conspicuous, 9F also had a higher HI compared with other plans, and significant difference was found only in 9F and 1A (P < 0.05). Figure 1 depicts the dose distribution of c-IMRT and VMAT in a cervical EC patient.
Figure 1.
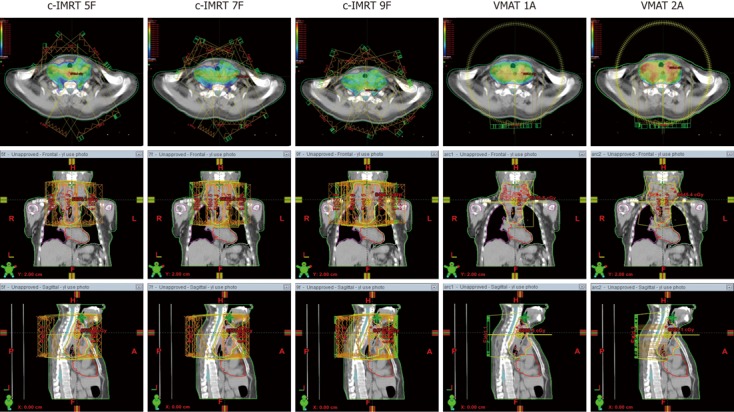
Dose distributions in a cervical esophageal cancer patient planed by conventional sliding window intensity-modulated radiotherapy (5 fields, 7 fields and 9 fields) and volumetric-modulated arc therapy (1 arc and 2 arcs). IMRT: Intensity-modulated radiotherapy; VMAT: Volumetric modulated arc therapy; F: Coplanar field; A: Arc; Orange line: Planning target volume; Blue line: Spinal cord; Color wash areas: Receiving ≥ 100% of the dose (60 Gy).
OAR
The absolute plan parameters for lungs, heart and spinal cord are summarized in Table 4. DVH of OAR in one patient were shown in Figure 2.
Table 4.
Dosimetric comparison for organs at risk of conventional sliding window intensity-modulated radiotherapy and volumetric-modulated arc therapy in cervical and upper thoracic esophageal cancer, and in middle and lower thoracic esophageal cancer, mean value (range)
| Organ | Variable | c-IMRT (5F, 7F, 9F) | VMAT (1A, 2A) | Relative reduction (%) | P < 0.05 |
| In cervical and upper thoracic | |||||
| Lung (Gy) | MLD1 | 12.65 (12.38-13.04) | 12.57 (12.35-12.79) | 0.6 | 2A vs 7F, 9F; 1A vs 7F |
| MLD2 | 14.35 (13.91-14.76) | 13.94 (13.74-14.14) | 2.9 | 2A vs 7F, 9F | |
| V51 | 48.52 (46.28-50.52) | 51.20 (51.03-51.37) | -5.5 | 1A, 2A vs 5F, 7F | |
| V52 | 61.53 (57.51-65.06) | 66.25 (66.07-66.43) | -7.7 | 1A , 2A vs 5F, 7F | |
| V101 | 39.30 (37.41-41.58) | 43.44 (43.23-43.65) | -10.5 | 1A , 2A vs 5F, 7F; 2A vs 9F | |
| V102 | 48.17 (44.18-52.07) | 54.25 (53.70-54.79) | -12.6 | 1A , 2A vs 5F, 7F, 9F | |
| V201 | 24.70 (23.93-25.16) | 24.17 (23.08-25.26) | 2.1 | 1A vs 5F | |
| V202 | 25.58 (24.29-26.23) | 22.85 (21.94-23.76) | 10.7 | NS | |
| V301 | 14.96 (14.42-15.33) | 12.99 (12.99-12.99) | 13.2 | 1A, 2A vs 5F, 7F, 9F | |
| V302 | 14.52 (14.27-15.00) | 12.01 (11.58-12.04) | 17.3 | 1A, 2A vs 5F, 7F; 2A vs 9F | |
| Heart (%) | V301 | 6.83 (5.7-7.41) | 5.30 (4.57-5.62) | 22.4 | NS |
| V302 | 15.20 (13.62-16.96) | 10.11 (9.98-10.24) | 33.5 | 1A vs 7F, 9F, 2A | |
| V401 | 4.33 (3.55-5.08) | 2.68 (2.45-2.91) | 38.1 | NS | |
| V402 | 8.32 (7.00-10.33) | 5.30 (5.08-5.22) | 36 | 1A vs 5F, 7F, 9F; 2A vs 5F | |
| V501 | 2.62 (2.07-3.09) | 1.44 (1.43-1.45) | 45 | NS | |
| V502 | 4.33 (3.44-5.53) | 2.63 (2.59-2.67) | 39.3 | NS | |
| Spinal cord (Gy) | Dmax1 | 37.92 (37.53-38.32) | 37.74 (37.31-38.17) | 0.5 | NS |
| Dmax2 | 37.85 (37.41-38.43) | 38.41 (38.05-38.76) | -1.5 | NS | |
| Head of humerus | Dmax1 | 10.00 (5.97-17.59) | 21.88 (20.80-22.95) | -118.8 | 1A , 2A vs 5F, 7F, 9F |
| (Gy) | Dmax2 | 7.57 (6.47-9.61) | 26.44 (26.35-26.52) | -249.3 | 1A , 2A vs 5F, 7F |
| Dmean1 | 3.24 (2.07-4.71) | 12.27 (11.85-12.69) | -278.7 | 2A vs 5F, 7F, 9F, 1A | |
| Dmean2 | 2.89 (1.47-4.86) | 15.26 (14.61-15.90) | -428 | 2A vs 5F, 7F, 9F, 1A | |
| In middle and lower thoracic | |||||
| Lung (Gy) | MLD3 | 15.03 (14.86-15.27) | 15.38 (15.24-15.51) | -2.3 | 1A, 2A vs 5F, 7F |
| MLD4 | 15.37 (15.01-15.81) | 15.67 (15.51-15.82) | -2 | 1A vs 5F | |
| V53 | 74.86 (71.00-79.05) | 82.83 (82.72-82.93) | -10.6 | 1A, 2A vs 5F, 7F, 9F | |
| V54 | 79.36 (72.43-85.85) | 89.91 (89.77-90.04) | -13.3 | 1A, 2A vs 5F, 7F, 9F | |
| V103 | 55.14 (52.64-58.66) | 65.26 (64.75-65.76) | -18.4 | 1A, 2A vs 5F, 7F, 9F | |
| V104 | 59.56 (54.27-64.76) | 72.54 (72.04-73.03) | -21.8 | 1A, 2A vs 5F, 7F; 2A vs 9F | |
| V203 | 24.39 (23.84-24.96) | 23.05 (22.28-23.81) | 5.5 | 2A vs 7F, 9F, 1A | |
| V204 | 25.32 (24.48-26.66) | 21.40 (20.69-22.11) | 15.5 | 1A, 2A vs 5F | |
| V303 | 12.64 (12.08-13.01) | 10.97 (10.88-11.06) | 13.2 | 1A,2A vs 5F, 7F; 2A vs 9F | |
| V304 | 11.13 (10.55-12.20) | 9.10 (8.58-9.62) | 18.2 | 1A, 2A vs 5F, 7F; 2A vs 9F | |
| Heart (%) | V303 | 47.30 (46.61-48.20) | 42.26 (40.72-43.79) | 10.7 | 1A vs 5F, 7F, 9F |
| V304 | 72.16 (67.81-78.60) | 56.55 (55.52-57.57) | 21.6 | 1A vs 5F, 7F, 9F; 2A vs 5F | |
| V403 | 26.89 (24.86-28.14) | 22.84 (20.98-24.69) | 15.1 | 1A vs 7F | |
| V404 | 33.53 (30.63-38.19) | 26.27 (26.21-26.32) | 21.7 | 2A vs 5F | |
| V503 | 14.06 (12.50-16.06) | 9.89 (8.93-10.85) | 29.7 | 1A vs 5F, 7F, 9F | |
| V504 | 17.99 (14.48-22.87) | 11.16 (10.93-11.39) | 38 | 1A, 2A vs 5F; 2A vs 7F, 9F | |
| Spinal cord (Gy) | Dmax3 | 39.03 (38.91-39.15) | 38.70 (38.54-38.86) | 0.8 | NS |
| Dmax4 | 37.61 (37.45-37.91) | 37.99 (37.78-38.20) | -1 | NS | |
Wilcoxon’s signed ranks test.
Cervical thoracic esophageal cancer;
upper thoracic esophageal cancer;
middle thoracic esophageal cancer;
lower thoracic esophageal cancer. c-IMRT: Conventional sliding window intensity-modulated radiotherapy; VMAT: Volumetric-modulated arc therapy; MLD: Mean dose of lungs; Dmax: Maximal dose; Dmean: Mean dose; F: Coplanar field; A: Arc; VX: The percentage of organ receiving a dose > X Gy; NS: Not significant.
Figure 2.
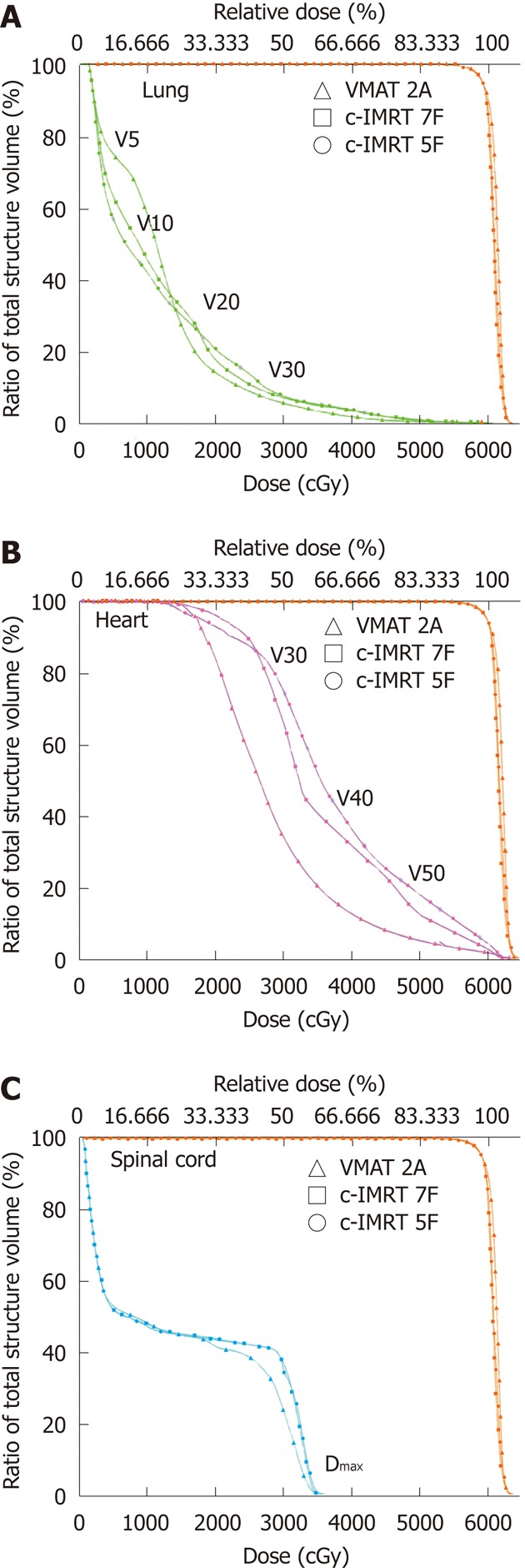
Dose-volume histogram of organs at risk and planning target volume for volumetric-modulated arc therapy and conventional sliding window intensity-modulated radiotherapy in a lower thoracic esophageal cancer patient. Volumetric-modulated arc therapy (VMAT) with double arcs (triangle) and conventional sliding window intensity-modulated radiotherapy (c-IMRT) with 7 fields (squares) and 5 fields (round). The planning target volume is shown in orange, the lungs in green (A), heart in red (B) and spinal cord in blue (C). F: Coplanar field; A: Arc; VX: The percentage of organ receiving a dose > X Gy.
The reduction trend of lung parameters (V5, V10, V20 and V30) was similar between the two techniques, except for MLD. In cervical and upper thoracic EC, MLD, V20 and V30 by VMAT were reduced by 0.6%-2.9%, 2.1%-10.7% and 13.2%-17.3%, respectively. V5 and V10 of lung by VMAT in cervical and upper thoracic EC were increased by 5.5%-7.7% and 10.5%-12.6%, respectively. In middle and lower thoracic EC, VMAT resulted in increased V5 (10.6%-13.3%), V10 (18.4%-21.8%) and MLD (2%-2.3%), but decreased V20 (5.5%-15.5%) and V30 (13.2%-18.2%). Statistically significant difference was found between VMAT (1A or 2A) and c-IMRT (5F, 7F and 9F) for V5, V10, V20 and V30, but for MLD, there was significant difference between VMAT (1A or 2A) and c-IMRT (5F, 7F) (P < 0.05).
For the heart, VMAT reduced V30, V40 and V50 as compared with c-IMRT, especially in thoracic cases. V30 by VMAT was reduced by 33.5%, 10.7% and 21.6%, V40 by 36%, 15.1% and 21.7%, and V50 by 39.3%, 29.7% and 38% in the upper, middle and lower thoracic EC, respectively. VMAT (1A or 2A) reduced V30 and V40 (5F, 7F and 9F) in thoracic EC, and V50 in middle and lower thoracic EC (P < 0.05). However, no difference was found in the Dmax of spinal cord between VMAT and c-IMRT.
NTCP results are shown in Table 5. VMAT (1A or 2A) significantly lowered the NTCP in comparison with c-IMRT (5F, 7F and 9F) in cervical and upper thoracic cases, while there was significant difference between 2A and 5F in middle and lower thoracic cases (P < 0.05). The trend of cardiac NTCP in VMAT was similar with lungs in thoracic EC, especially in middle and lower thoracic EC (P < 0.05).
Table 5.
Normal tissue complication probability results for organs at risk
| Organ | IMRT-5F | IMRT-7F | IMRT-9F | VMAT-1A | VMAT-2A | P < 0.05 |
| Lung | ||||||
| Cervical | 0.24 ± 0.14 | 0.24 ± 0.15 | 0.32 ± 0.22 | 0.22 ± 0.18 | 0.13 ± 0.09 | 1A vs 9F; 2A vs 5F, 7F, 9F |
| Upper | 0.73 ± 0.62 | 0.77 ± 0.66 | 0.84 ± 0.71 | 0.41 ± 0.37 | 0.30 ± 0.23 | 1A vs 7F, 9F; 2A vs 5F, 7F, 9F |
| Middle | 0.62 ± 0.48 | 0.59 ± 0.48 | 0.60 ± 0.47 | 0.57 ± 0.45 | 0.50 ± 0.41 | 2A vs 5F |
| Lower | 0.59 ± 0.45 | 0.59 ± 0.50 | 0.67 ± 0.46 | 0.57 ± 0.54 | 0.46 ± 0.37 | 2A vs 5F |
| Heart | ||||||
| Cervical | 0 | 0 | 0 | 0 | 0 | NS |
| Upper | 0.02 (0-0.12) | 0.02 (0-0.09) | 0 (0-0.02) | 0 | 0 | NS |
| Middle | 0.61 (0.05-1.07) | 0.94 (0.01-4.24) | 0.31 (0.01-1.17) | 0.13 (0-0.58) | 0.21 (0-0.80) | 1A vs 5F, 7F, 9F |
| Lower | 6.66 (1.14-16.04) | 1.97 (0.11-5.09) | 1.76 (0.07-5.95) | 1.32 (0-5.81) | 0.66 (0-1.59) | 1A vs 5F, 9F; 2A vs 5F, 7F, 9F |
| Spinal cord | ||||||
| Cervical | 0 | 0 | 0 | 0 | 0 | NS |
| Upper | 0 | 0 | 0 | 0 | 0 | NS |
| Middle | 0.002 (0-0.01) | 0.006 (0-0.03) | 0.008 (0-0.04) | 0 | 0 | NS |
| Lower | 0 | 0 | 0 | 0 | 0 | NS |
IMRT: Intensity-modulated radiotherapy; VMAT: Volumetric modulated arc therapy; F: Coplanar field; A: Arc; NS: Not significant.
It was worth noting that Dmean and maximal doses to the humeral head (HHmean and HHmax) in VMAT were dramatically increased in comparison with c-IMRT in cervical and upper thoracic EC (Table 4). Compared with c-IMRT, HHmean in VMAT was increased by almost three times in cervical EC and four times in upper thoracic EC (P < 0.05). HHmax in VMAT was twice higher in cervical EC and three times higher in upper thoracic EC than that in c-IMRT (5F and 7F) (P < 0.05).
Monitor units
The c-IMRT plans required an increased MU per fraction when the field was increased whereas the VMAT plans usually resulted in lower MU when rotating arcs were increased. 1A plans required at least 50% or 60% less than 9F in cervical and upper EC or middle and lower EC (P < 0.05). The difference between VMAT (1A and 2A) and c-IMRT (5F, 7F and 9F) remained significant (P < 0.05) in all the cases. Detailed information about MU is shown in Table 3.
DISCUSSION
In the present study, VMAT proved to be slightly better than c-IMRT for targeting dose distribution in EC of all locations, and to have equivalent or better OAR dose sparing and lower NTCP. We initiated a dosimetric and radiobiological comparison in the EC of all locations in this study. The results indicated that VMAT could generate better radiotherapeutic plans than sliding window IMRT.
VMAT is a complex form of IMRT that allows dose delivery in single or multiple arcs. Two arcs allowed superior modulation factor during optimization due to the independent optimization, and unrelated sequence of MLC shape, gantry speed and dose rate combinations. This approach provided adequate coverage of PTV and spare of OARs at least equivalent to c-IMRT, while it could reduce significantly the treatment time and the number of MU required in the morbidities, such as head and neck cancer, intracranial tumor, breast cancer, glioma, and carcinoma of the anal canal[14,17,20,21]. In the present study, VMAT using 2A achieved better results than using 1A.
First, the PTV volumes were larger in cervical and upper EC series due to pathological characteristics and biological behavior of carcinoma in these regions, and presented T-shaped from a posteroanterior view. In head and neck carcinoma, due to more complex target volume, 7F or 9F are constantly used in c-IMRT to meet the requirements of dose distribution, HI and CI of PTV[22,23]. Our results were consistent with this. PTV coverage in c-IMRT with 5F was less qualified in comparison with 7F or 9F. Both VMAT and c-IMRT resulted in abundant Dmean in PTV (63.94 Gy and 63.53 Gy in 1A and 2A vs 63.49 Gy, 62.90 Gy, 62.46 Gy in 5F, 7F and 9F). VMAT proved to be superior to c-IMRT in terms of MU and CI, but slightly inferior to c-IMRT in terms of HI. We also confirmed that VMAT with 2A achieved better results than 1A in terms of conformity and homogeneity. For the heart, VMAT showed a lower percentage of V30, V40 or V50. For lungs, VMAT provided better sparing in terms of V20 and V30. The results of radiobiological NTCP comparison demonstrated that VMAT was superior to c-IMRT either in lungs or heart (P < 0.05). Yin et al[24] compared 7F in IMRT with VMAT plans for cervical EC, and found that there were differences between VMAT and IMRT in HI and MU, but not in CI, which are consistent with our results, but lung V5 in VMAT (1A 51.4, AX 49.3 and 7F 50.9) was reduced while lung V30, V40, V50 and MLD were increased. In our study, lung V5 in VMAT was slightly increased (1A 51.37, 2A 51.03, and 7F 48.77), but V30 (1A 12.99, 2A 12.99, 7F 14.42) and MLD (1A 12.79, 2A 12.35 7F 12.52) were lower than in c-IMRT. The difference in V5, V30 and MLD may be due to that they avoided a certain angle in the VMAT plan with 2A, and this caused the reduction of the volume of irradiated lungs. One of the particular interesting phenomena in VMAT is the increase trends of mean or maximal radiation doses to the humeral head. This may be because that humeral head is adjacent to the target volume in the cervical and upper EC and the rotating mode of VMAT thus increased the irradiation volumes of the humeral head. However, clinical evidence on the acceptable humeral head constraints for IMRT remains scarce in literature. Nevertheless, according to the tolerated doses and clinical data of bone joints, such as femoral head and neck or temporomandibular joint[25,26], the maximum doses to humeral heads in the cervical EC (1A 34.00 Gy and 2A 33.82 Gy) or upper thoracic EC (1A 44.33 Gy and 2A 41.03 Gy) were considered acceptable, but we should pay attention to this performance and its potential risk.
Subsequently, the results obtained in cervical and upper thoracic EC were almost seen in middle and lower thoracic EC, with a PTV of smaller volume but surrounded by more lungs and heart. In thoracic and epigastric cases, except for a few complex cases, 5-7F of c-IMRT could meet most of the clinical dosimetric requirements. In our study, an ideal homogeneity was achieved in 5F or 7F of IMRT. With increase of the fields in c-IMRT or doubling arc in VMAT, dose distribution in PTV became more optimal in terms of better conformity and similar homogeneity. The trends were significantly different (P < 0.05) between VMAT(1A and 2A) and c-IMRT(5F and 7F). Recently, Van Benthuysen et al[27] demonstrated that VMAT had the advantage to decrease treatment times over c-IMRT, while providing similar OAR sparing and PTV coverage, but lower homogenous dose distribution in lower EC. In our study, we found that in the middle and lower thoracic EC, HI was similar in VMAT and c-IMRT. We did not find a significant difference between 5F or 7F IMRT and VMAT for these trends. For lesions in this region, more volumes of heart and lungs were involved in the irradiation area, mean doses to lungs and heart were elevated markedly and HI was also inferior to cervical and upper EC. Because lung tissue filled with air was significantly less dense than other body tissues, as a result of heterogeneity corrections in radiation treatment planning systems, optimization procedures produced substantial dose non-uniformity in PTV caused by the effect of surrounding lung tissues. To further optimize the dose in the target volume, dose heterogeneity was achieved by loosening the constraints on the maximum doses in PTV. It may result in insufficient dose in PTV or the creation of clinically significant hotspots in the PTV and surrounding normal tissue structures. The National Comprehensive Cancer Network Guidelines recommend dose limits for selecting critical normal structures, i.e., the spinal cord doses should not exceed 45 Gy, and one-third of the heart should receive less than 50 Gy. The dosimetric parameters of lung injury risk were mainly studied on lung cancer irradiation, the increased risk of radiation pneumonitis correlated with heterogeneous parameters, such as MLD, the percentage of lung volume receiving at least 20 Gy (V20), 13 Gy (V13), 10 Gy (V10) or 5 Gy (V5), in which V20 was a recognized indicator confirmed by several studies. Based on pooled data from 540 patients irradiated for thoracic malignancy, the calculated risk of grade ≥ 2 pneumonitis was 43%, 18%, and 11% for the MLD of 24-36, 16-24 and 8-16 Gy, respectively. In our study, MLD was controlled below 16 Gy and it was acceptable. For conventionally fractionated regimens (2 Gy/fraction), V20 and MLD were the traditional parameters used to predict for lung toxicity, however, emerging data suggested that percentage of lung volume receiving lower doses may be predictive of pulmonary toxicity. VMAT plans offered the potential to significantly escalate the coverage of the low-dose area (V5 and V10) because all doses were deposited within the plane of the arc, instead of being spread out in non-coplanar directions. Mean V5 in VMAT was beyond 80% and it might increase the potential pulmonary toxicity. Wei et al[28] found that V30 > 46% and < 46% was associated with rates of pericardial effusion of 73% and 13%, respectively. The ischemic segments usually occur in volumes irradiated to a dose of 45 Gy or more. In our study, V40 and V50 were achieved in both VMAT and c-IMRT, but V30 was higher due to lower constrained priority. In VMAT, 1A achieved better results than 2A for less irradiation volumes of heart and lungs. In comparison with 5F or 7F, VMAT reduced V20 and V30 of lungs, and V30, V40 and V50 of heart. Besides, Hawkins et al[29] evaluated the capability of VMAT to reduce heart and cord dose while maintaining lung V20 < 20% in lower gastroesophageal tumors. IMRT (4F) and VMAT plans showed that VMAT provided a significant reduction in heart V30 (31% vs 55%) with a better CI in a shorter time. But V30 (2A 57.57 vs 5F 78.6) in our study was higher because our prescribed dose (60 Gy) was higher than theirs (54 Gy). NTCP of heart and lungs were common indicators in radiobiological assessment to indicate the tendency in plan comparison. VMAT had a trend with a lower NTCP of lungs and heart, but statistical significance only existed for lungs between 5F and 2A in lower EC, as for heart, between c-IMRT and 1A in middle thoracic EC, and between c-IMRT and 2A in lower EC (P < 0.05). Therefore, regarding the correlation between dosimetric parameters and OAR toxicity, we did find a superior trend in VMAT to c-IMRT. Wang et al[30] also conducted a planning comparison for EC between VMAT (1A and 2A) and 7F IMRT, and found that VMAT plan, especially using double arcs, could improve OAR sparing (lung V20 and V30, heart V30 and V40) and lower MUs without compromised target qualities as compared with IMRT. This was consistent with our findings.
VMAT reduced the number of required MU[31], because it was performed simultaneously with rotation by a dynamic MLC adaptation to the target volume during the rotation. Using double arcs, the rotation in clock-wise and counter clock-wise directions allows diminished 25 s off-time between the two arcs[32]. The number of MU required is higher due to the sliding window technique. c-IMRT plans in this study offered wider than 15 cm in the direction of the MLC motion necessitating splitting into two sequences and doubling the number of fields. By contrast, one of the drawbacks of c-IMRT was the potential risk of second cancer. Theoretically, the significant reduction of MU by VMAT decreases scattered dose and may reduce the risk of secondary malignancies. The impact of irradiation of healthy tissues at low doses remains unresolved with the use of VMAT.
In conclusion, VMAT treatment plan was slightly better than c-IMRT in terms of PTV coverage. It provided an equivalent or better lungs and heart dose sparing, significant reduction of NTCP and MU per fraction. For cervical and upper EC, PTV was T-shaped across neck and chest, VMAT achieved fairly uniform dose distribution, but the 2A provided the best CI in all plans, and VMAT significantly increased the doses of humeral head. For middle and lower EC, in which PTV involved more lungs, VMAT plans offered the most conformal dose distribution and the potential to significantly escalate the coverage of lungs at low doses.
COMMENTS
Background
Esophageal cancer (EC) is very common in China and other developing countries. Radiotherapy is a major non-invasive treatment method with a high efficacy rate for EC. Innovative technologies have been developed in radiation delivery such as intensity-modulated radiotherapy (IMRT), and volumetric modulated arc therapy (VMAT) is a relatively new form of IMRT. There are several new interesting techniques in IMRT, but few evaluations have been available in term of their efficiency and safety.
Research frontiers
There have been few studies to compare the two radiotherapy techniques, particularly in EC. VMAT had already been investigated for some cancers. In this study, the authors further compared the VMAT plans and the conventional sliding window IMRT plans in EC of different anatomic regions.
Innovations and breakthroughs
Previous dose comparison studies showed that VMAT was able to produce similar or improved dose distributions, while achieving a reduction in treatment time and monitor units (MU). However, results are different in EC about dose to organs at risk (OAR) due to variable limitation conditions. Normal tissue complication probability (NTCP) of OAR in the VMAT plan, a common indicator in radiobiological assessment, is still not clear in EC. In the present study, the authors showed that VMAT, especially 2 arcs, slightly improved the OAR dose sparing for some organs, such as lungs and heart, and reduced the NTCP and MU with a better planning target volume coverage.
Applications
This study provides a new insight into better understanding of the VMAT plan characteristics in EC of different anatomical parts, and lays the foundation for further clinical studies in VMAT.
Terminology
IMRT is a three-dimensional conformal radiotherapy developed based on inverse planning optimization to modulate intensity beams using multi-leaf collimator (MLC), this technique offers improvement in target dosimetric coverage. There are different IMRT delivery techniques, including “step and shoot”, sliding window modes and a rotational technique (VMAT). Conventional sliding window IMRT, in which the leaves are adjusted with fixed gantry, is a common form which is being used in clinical practice. VMAT, in which dose rates, gantry speed and dynamic MLC motion are all variable during gantry arc rotation, is a novel form of IMRT in recent years.
Peer review
IMRT is developing a lot in Radiation Oncology Departments for a few years. There are several very interesting technics but still few real evaluations in terms of efficiency and safety. Comparing two technics is very interesting even if it’s “only” a dosimetric comparison. This kind of comparisons of recent technics are not very frequent, particularly in EC but are developing a lot this last few years. The text of this manuscript is very clear and well presented.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30870738
Peer reviewer: Stéphane Supiot, MD, PhD, Department of Radiation Oncology, Centre René Gauducheau, St-Herblain, 44800 Nantes, France
S- Editor Lv S L- Editor A E- Editor Zheng XM
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Rochet N, Kieser M, Sterzing F, Krause S, Lindel K, Harms W, Eichbaum MH, Schneeweiss A, Sohn C, Debus J. Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III--the OVAR-IMRT-02 Study. BMC Cancer. 2011;11:41. doi: 10.1186/1471-2407-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirix P, Vanstraelen B, Jorissen M, Vander Poorten V, Nuyts S. Intensity-modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:998–1004. doi: 10.1016/j.ijrobp.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 4.Orlandi E, Palazzi M, Pignoli E, Fallai C, Giostra A, Olmi P. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: A review. Crit Rev Oncol Hematol. 2010;73:111–125. doi: 10.1016/j.critrevonc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:643–657. doi: 10.1016/j.clon.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Pederson AW, Fricano J, Correa D, Pelizzari CA, Liauw SL. Late toxicity after intensity-modulated radiation therapy for localized prostate cancer: an exploration of dose-volume histogram parameters to limit genitourinary and gastrointestinal toxicity. Int J Radiat Oncol Biol Phys. 2012;82:235–241. doi: 10.1016/j.ijrobp.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol. 1995;40:1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 8.Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty U, Engineer R, Deshpande DD, Jamema SV, Vanetti E, Clivio A, Nicolini G, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, Herskind C, Polednik M, Steil V, Wenz F, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald TJ, Moser R. Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78:1457–1466. doi: 10.1016/j.ijrobp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys. 2010;76:446–451. doi: 10.1016/j.ijrobp.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 12.Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer. 2010;29:843–854. doi: 10.5732/cjc.010.10151. [DOI] [PubMed] [Google Scholar]
- 13.Esophageal Carcinoma Cooperative Group of Radiation Oncology Society of Chinese Medical Association. Treatment guideline of radiotherapy for Chinese esophageal carcinoma (draft) Chin J Cancer. 2010;29:855–859. [PubMed] [Google Scholar]
- 14.Fogliata A, Clivio A, Nicolini G, Vanetti E, Cozzi L. Intensity modulation with photons for benign intracranial tumours: a planning comparison of volumetric single arc, helical arc and fixed gantry techniques. Radiother Oncol. 2008;89:254–262. doi: 10.1016/j.radonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Fiorino C, Rancati T, Valdagni R. Predictive models of toxicity in external radiotherapy: dosimetric issues. Cancer. 2009;115:3135–3140. doi: 10.1002/cncr.24354. [DOI] [PubMed] [Google Scholar]
- 16.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer R, Nichol AM, Vollans E, Fong M, Nakano S, Moiseenko V, Schmuland M, Ma R, McKenzie M, Otto K. A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas. Int J Radiat Oncol Biol Phys. 2010;76:1177–1184. doi: 10.1016/j.ijrobp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Miller J, Fuller M, Vinod S, Suchowerska N, Holloway L. The significance of the choice of radiobiological (NTCP) models in treatment plan objective functions. Australas Phys Eng Sci Med. 2009;32:81–87. doi: 10.1007/BF03178632. [DOI] [PubMed] [Google Scholar]
- 19.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 20.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, Wai ES, Otto K. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, Cozzi L. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:118–124. doi: 10.1016/j.radonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74:252–259. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava SP, Das IJ, Kumar A, Johnstone PA. Dosimetric comparison of manual and beam angle optimization of gantry angles in IMRT. Med Dosim. 2011;36:313–316. doi: 10.1016/j.meddos.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, Chen J, Xing L, Dong X, Liu T, Lu J, Yu J. Applications of IMAT in cervical esophageal cancer radiotherapy: a comparison with fixed-field IMRT in dosimetry and implementation. J Appl Clin Med Phys. 2011;12:3343. doi: 10.1120/jacmp.v12i2.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen I, Carl J, Lund B, Larsen EH, Nielsen J. Radiobiological impact of reduced margins and treatment technique for prostate cancer in terms of tumor control probability (TCP) and normal tissue complication probability (NTCP) Med Dosim. 2011;36:130–137. doi: 10.1016/j.meddos.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Kehwar TS. Analytical approach to estimate normal tissue complication probability using best fit of normal tissue tolerance doses into the NTCP equation of the linear quadratic model. J Cancer Res Ther. 2005;1:168–179. doi: 10.4103/0973-1482.19597. [DOI] [PubMed] [Google Scholar]
- 27.Van Benthuysen L, Hales L, Podgorsak MB. Volumetric modulated arc therapy vs. IMRT for the treatment of distal esophageal cancer. Med Dosim. 2011;36:404–409. doi: 10.1016/j.meddos.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Wei X, Liu HH, Tucker SL, Wang S, Mohan R, Cox JD, Komaki R, Liao Z. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins MA, Bedford JL, Warrington AP, Tait DM. Volumetric modulated arc therapy planning for distal oesophageal malignancies. Br J Radiol. 2012;85:44–52. doi: 10.1259/bjr/25428720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Yang Y, Zhu J, Li B, Chen J, Yin Y. 3D-conformal RT, fixed-field IMRT and RapidArc, which one is better for esophageal carcinoma treated with elective nodal irradiation. Technol Cancer Res Treat. 2011;10:487–494. doi: 10.7785/tcrt.2012.500225. [DOI] [PubMed] [Google Scholar]
- 31.Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, Upreti RR, Budrukkar A, Murthy V, Deshpande DD, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:111–117. doi: 10.1016/j.radonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Vieillot S, Azria D, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, Aillères N, Fenoglietto P. Plan comparison of volumetric-modulated arc therapy (RapidArc) and conventional intensity-modulated radiation therapy (IMRT) in anal canal cancer. Radiat Oncol. 2010;5:92. doi: 10.1186/1748-717X-5-92. [DOI] [PMC free article] [PubMed] [Google Scholar]


