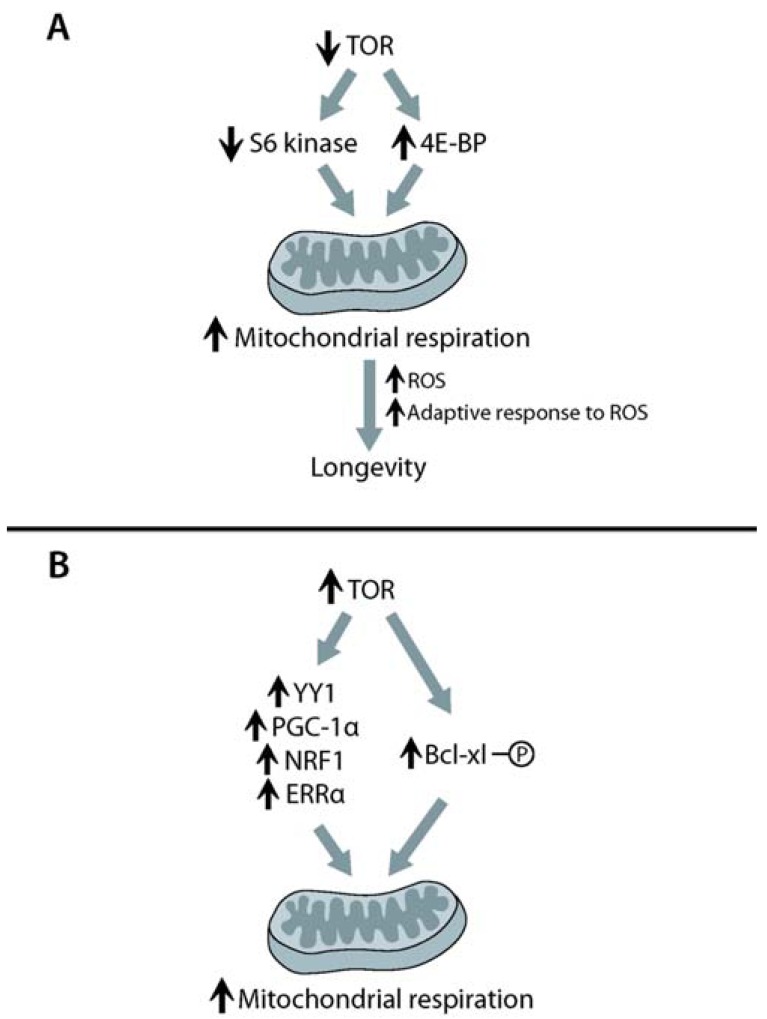
Fig. (3).
Two contradicting models of the effects of TOR (target of rapamycin) signaling on mitochondrial function. (A) Reduced TOR signaling increases mitochondrial respiration to induce longevity. Inhibition of TOR signaling downregulates ribosomal protein S6 kinase and upregulates 4E-BP. This leads to overall increase in mitochondrial respiration that may elevate the level of ROS (reactive oxygen species). The production of ROS is likely to confer an adaptive response that is required for longevity. (B) TOR signaling positively regulates mitochondrial function. Activated TOR upregulates YY1 (yin-yang 1), PGC-1α, NRF1 (nuclear respiratory factor-1) and ERRα (estrogen-related receptor α), key transcription factors that increase mitochondrial function. YY1 directly binds PGC-1α and TOR to increase the TOR-dependent expression of mitochondrial genes. In addition, TOR phosphorylates Bcl-xl (B-cell lymphoma-extra large), an outer membrane protein of mitochondria, to enhance mitochondrial respiration. It is currently unknown whether these processes shown in (B) are involved in lifespan regulation.