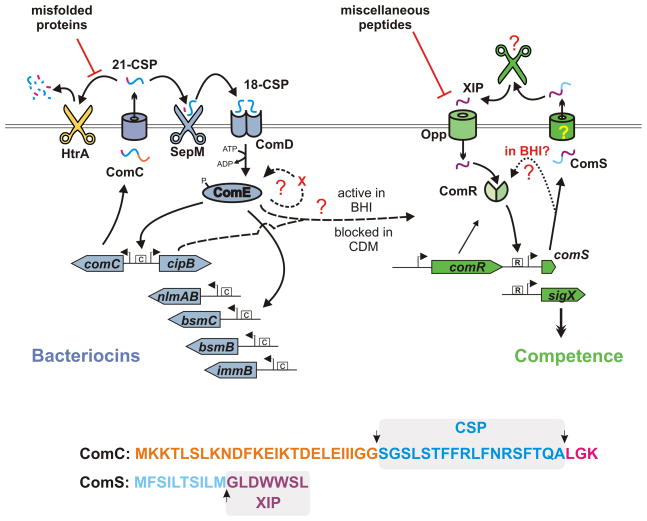
Figure 1.
Model of CSP and XIP quorum sensing in Streptococcus mutans. ComC (whose sequence is included at the bottom and color coded to illustrate processing sites) is secreted as a pre-peptide termed 21-CSP. CSP can be degraded by HtrA in a process affected by misfolded proteins. Productive processing by SepM generates the mature signal, 18-CSP, that binds to ComD leading to phosphorylation of ComE. ComE-P binds inverted repeat sites, indicated by a boxed “C”, enhancing transcription of bacteriocin genes. A positive feedback loop affecting ComE expression is proposed to include an additional regulatory component that establishes a bimodal expression pattern. ComS is secreted and processed by unknown components, but the mature form of the pheromone, XIP, comprises the last seven amino acids of ComS. XIP is imported via the oligopeptide permease (Opp), but transport is blocked by competing peptides present in complex media. Within the cell, an XIP-ComR complex binds to sites indicated by boxed “R” elements, activating transcription of comS and sigX. ComR and ComS are required to induce SigX, and SigX is required for transformation. In the report by Son et al. (Son et al., 2012), medium components are shown to modulate both CSP and XIP systems. In CDM, CSP does not induce competence, while XIP does so efficiently. In BHI, CSP stimulates competence, but, paradoxically, XIP signaling is blocked, yet comS remains required to stimulate sigX. For this reason it is proposed that ComS bypasses secretion and processing to interact with ComR directly.