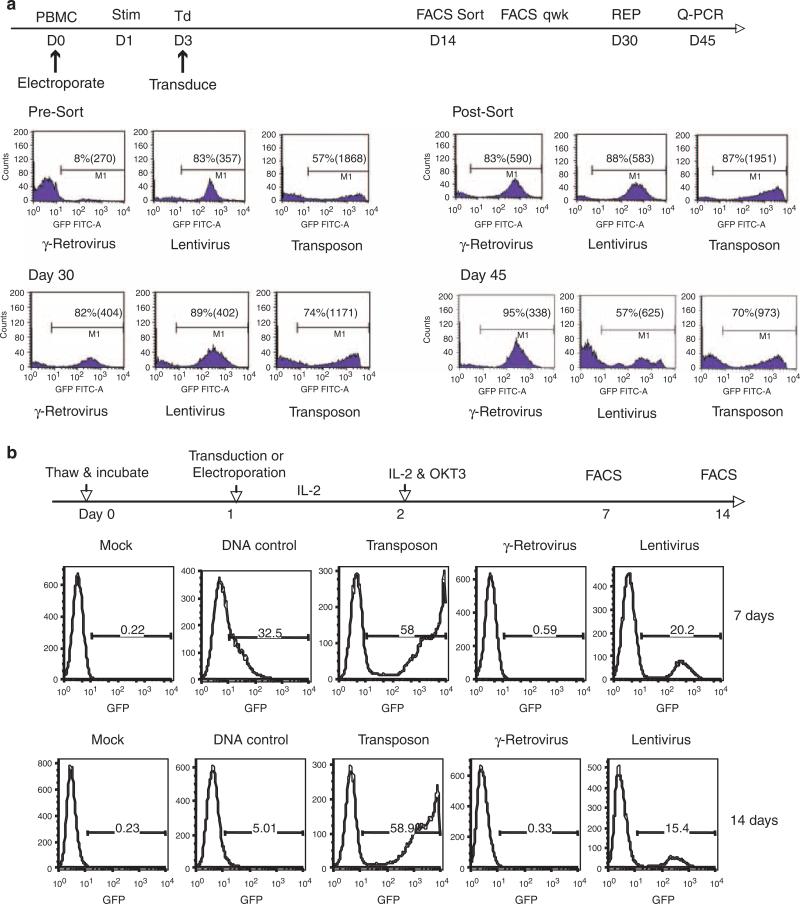
Figure 2.
Comparison of green fluorescent protein (GFP) reporter expression in γ-retroviral, lentiviral or transposon-mediated genetically modified PBL. Identical expression gene expression cassettes (MSCV-U3-GFP) were cloned into each vector system and produced as described.40,41 (a) Peripheral blood mononuclear cell (PBMC) were electroporated with transposon system on day 0 or transduced with viral vectors following 2 days of stimulation with interleukin-2 (IL2; 300 IU ml-1) and OKT3 (50 ng ml-1). On day 14, GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS) to normalize for differences in gene transfer efficiency. GFP-positive population of cells in each vector group was assayed weekly by FACS and on day 30 subject to a further expansion using the rapid expansion protocol (REP).42 Shown were the percent positive cells and mean fluorescence intensity (MFI) of each group measured at the times indicated. Transgene copy number was determined by quantitative PCR after normalization with β-actin. β-Actin and GFP primer-probes (Tamra) were from Applied Biosystems Oligonucleotide Factory (Foster City, CA, USA). Reactions performed on Applied Biosystems 7500 Fast Real-time PCR System. (b) Cryopreserved PBMC were resuspended in AIM-V medium and culture overnight. The next day, cells were subject to electroporation with the Sleeping Beauty (SB) transposon alone (DNA control) or transposon plus transposase RNA (Transposon) or transduction with γ-retroviral or lentiviral vectors. IL2 (300 IU ml-1) was added to the cultures after gene transfer and the following day T cells were stimulated with OKT3 (50 ng ml-1). The number GFP-positive cells in each vector group were determined by FACS at the time points indicated.