Table I.
Synthesis and Binding Affinity of Novel Pirenzepine Analogues. All Compounds Were Analyzed for C, H, and N within ±0.4%, Except Where Noted. Ki Values (±sem) for Inhibition of Binding of [3H]-N-Methylscopolamine Are Given for m1–m4 Muscarinic Receptors, for One to Three Determinations, Each Performed in Triplicate. R″ = H, Except for Compound 17 As Indicated. Binding Was Carried Out for 90 min at 37 °C, Using Membranes from Rat Heart Tissue (m2 Receptors) and Transfected A9L Cells (m1 and m3 Receptors) and in NG108-15 Cells (m4 Receptors) in the Presence of 0.5 nM [3H]-NMS. Ki Values Are Expressed in Units of Micromolar
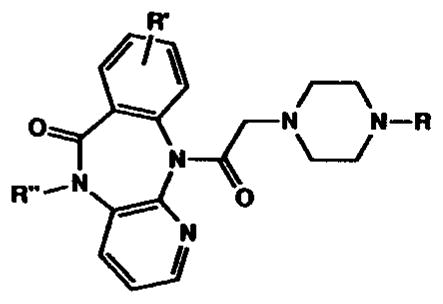
| ||||||||
|---|---|---|---|---|---|---|---|---|
| compd | R = | R′ = | mp, °C | Anal. | m1 | m2 | m3 | m4 |
| 1 | CH3 | H | 0.029 | 0.61 ± 0.05 | 0.24 | 0.33 | ||
| 3 | 0.0018 | 0.0503 | 0.0069 | 0.0174 | ||||
| 11a | CH3 | 8-SCH3 | >205dec | C20H23N5O2S·1HCl·1.5H2O | 4.66 ± 1.02 | 11.0 | 3.62 ± 0.14 | n.d.l |
| 11b | CH3 | 8-SO2NH2 | C19H22N6O4S·0.5H2Oa | >30 | >30 | >30 | n.d. | |
| 11c | CH3 | 8-SO2NH(CH2)2NHBoc | 210–213 dec | C26H35N7O6S·3oxalate·0.25H2O | >30 | n.d. | >30 | n.d. |
| 11d | CH3 | 8-SO2NH(CH2)2NH2 | 155–158 | C21H27N7O4S·1.5oxalate·0.5H2O | >30 | n.d. | >30 | n.d. |
| 11e | CH3 | 8-SO2NH(CH2)2NHAc | 190–193 | C23H29N7O5S·2oxalate·0.5H2O | >30 | n.d. | >30 | n.d. |
| 15 | CH3 | 9-CH2OSi(CH3)2-t-Bu | 245–248 | C26H37N5O3Si·0.25H2O | >30 | 4.4 ± 0.3 | >30 | n.d. |
| 16 | CH3 | 9-CH2OH | C20H23N5O3·1.5H2O | 4.4 | n.d. | 4.4 | n.d. | |
| 17aj | CH3 | H | 108–111 | C21H25N5O2·1HCl·1.75H2O | 7.7 ± 2.1 | 8.00 | n.d. | n.d. |
| 17bk | CH3 | H | oil | C26H32N6O2·0.5H2O | 8.9 ± 4.7 | n.d. | n.d. | n.d. |
| 18 | H | H | >230 | 0.37 | 3.34 | 1.31 | 1.67 | |
| 19a | COCH3 | H | >290 | C20H21N5O3·0.25H2O | >100 | >100 | >100 | >100 |
| 19b | COC6H5 | H | 202–205 | C25H23N5O3·HCl·0.75H2O | >100 | >100 | >100 | >100 |
| 20 | SO2CH3 | H | 180–183 | C19H21N5O4S·HCl | >100 | >100 | >100 | >100 |
| 21 | CSNHC6H5 | H | 204–206 | C25H24N6O2S·0.75H2O | >100 | >100 | >100 | >100 |
| 22 | CH2≡CH | H | 249–251 | C21H21N5O2 | 1.67 ± 0.06 | 1.37 ± 0.06 | n.d. | n.d. |
| 23 | CH2C6H5 | H | 145–148 | C25H25N5O2·oxalate·H2Ob | 0.33 | 0.24 ± 0.10 | 0.24 | 0.22 |
| 24a | CH2C6H4-p-CONHCH3 | H | 191–193 | C27H28N6O3·oxalate·0.5H2O | 2.84 | 2.65 | 4.02 | 2.94 |
| 24b | CH2C6H4-p-CONH(CH2)2NHBoc | H | 155–158 | C33H39N7O5·oxalate·H2Oc | n.d. | 2.50 | n.d. | n.d. |
| 24c | CH2C6H4-p-CONH(CH2)2NH2 | H | 208–212 | C26H31N7O3·oxalate·5H2Od | 1.39 | 0.96 | 3.65 | 1.04 |
| 24d | CH2C6H4-p-CONH(CH2)4NHBoc | H | 173–175 | C35H43N7O5·oxalate·0.5H2O | 1.68 | 1.09 | 3.47 | n.d. |
| 25 | (CH2)2OH | H | 197–200 | C20H23N5O3 | 5.8 ± 1.0 | 3.2 | 9.5 | n.d. |
| 26 | (CH2)2Cl | H | 188–190 | C20H22N5O2Cl·2HCl·1.5H2O | 0.052 | 0.21 | 0.078 | 0.13 |
| 27 | (CH2)2NH2 | H | 190–193 | C20H24N6O2·1H2O | 1.19 | 3.04 | 1.97 | 2.84 |
| 28 | (CH2)2NHAc | H | 93–95 | C22H26N6O3·0.75H2O | 0.67 | 2.52 | 0.97 | 2.57 |
| 29a | (CH2)2NHCO(CH2)2NHBoc | H | 112–115 | C28H37N7O5·1CHCl3·0.5H2O | 13.3 ± 5.95 | 6.06 | 6.19 ± 0.28 | n.d. |
| 29b | (CH2)2NHCO(CH2)3NHBoc | H | 130–133 | C29H39N7O5e | 4.80 ± 1.36 | 3.34 | 4.10 ± 0.52 | n.d. |
| 30a | (CH2)2NHCO(CH2)2NH2 | H | 85–88 | C23H29N7O3·2.67TFA·1.55H2O | 4.34 ± 0.26 | 5.84 | 6.65 ± 0.43 | n.d. |
| 30b | (CH2)2NHCO(CH2)3NH2 | H | C24H31N7O3·3TFA | 9.53 ± 2.46 | 6.34 | 11.5 ± 1.98 | n.d. | |
| 31a | (CH2)2NHCO(CH2)2NHAc | H | 180–183 | C25H31N7O4·0.75H2O | 39.4 ± 16.6 | 34.5 | >100 | n.d. |
| 31b | (CH2)2NHCO(CH2)3NHAc | H | 162–165 | C26H33N7O4·2.75H2Of | 9.65 ± 2.98 | 8.82 | 10.8 ± 0.45 | n.d. |
| 32d | (CH2)6NHBoc | H | C29H40N6O4·oxalate·H2Og | 0.0371 ± 0.0017 | 0.0214 ± 0.0039 | 0.0460 ± 0.0155 | 0.0232 ± 0.0053 | |
| 33a | (CH2)3NPth | H | 202–204 | C29H28N6O4·0.75H2O | 1.81 ± 0.40 | 0.84 ± 0.35 | n.d. | n.d. |
| 33b | (CH2)4NPth | H | 195–196 | C30H30N6O4·oxalate·0.5H2O | 0.17 ± 0.006 | 0.089 ± 0.005 | 0.12 | 0.21 |
| 33c | (CH2)5NPth | H | 120–122 | C30H32N6O4 | 0.060 ± 0.001 | 0.020 ± 0.001 | n.d. | n.d. |
| 33d | (CH2)6NPth | H | 157–158 | C32H34N6O4·1DMF | 0.018 ± 0.008 | 0.018 ± 0.003 | n.d. | n.d. |
| 34a | (CH2)3NH2 | H | 111–113 | C21H26N6O2·1.5H2O | 3.87 ± 0.12 | 5.12 ± 0.93 | n.d. | n.d. |
| 34b | (CH2)4NH2 | H | 121–123 | C22H28N6O2·1H2O | 3.08 ± 0.22 | 1.74 ± 0.03 | n.d. | n.d. |
| 34c | (CH2)5NH2 | H | 200–202 | C23H30N6O2·1H2O | 1.17 ± 0.01 | 5.89 ± 0.13 | n.d. | n.d. |
| 34d | (CH2)6NH2 | H | 138–140 | C24H32N6O2·0.5CHCl3·0.67H2Oh | 1.31 ± 0.02 | 1.34 ± 0.12 | n.d. | n.d. |
| 34e | (CH2)7NH2 | H | 72–75 | C25H34N6O2·1.5-H2O | 0.066 | 0.057 | n.d. | n.d. |
| 34f | (CH2)8NH2 | H | 65–68 | C26H36N6O2·H2O | 0.020 ± 0.001 | 0.026 ± 0.0005 | n.d. | n.d. |
| 34g | (CH2)9NH2 | H | 140–143 | C27H38N6O2 | 0.024 ± 0.001 | 0.024 ± 0.007 | n.d. | n.d. |
| 34h | (CH2)10NH2 | H | 95–97 | C28H40N6O2·1.5H2O | 0.0158 ± 0.0009 | 0.0117 ± 0.0007 | n.d. | n.d. |
| 35g | (CH2)9NHAc | H | 170–173 | C29H40N6O3·2oxalate | 0.052 ± 0.001 | 0.050 ± 0.018 | n.d. | n.d. |
| 35h | (CH2)10NHAc | H | 87–90 | C30H48N6O3·0.5H2O | 0.075 ± 0.013 | 0.043 ± 0.025 | n.d. | n.d. |
| 36 | (CH2)10NEt2 | H | 168–171 | C32H48N6O2·4oxalate·3H2O | 0.0030 ± 0.0004 | 0.0135 ± 0.0009 | n.d. | n.d. |
| 37 | (CH2)10NHCH2-(o-MeO)Ph | H | 153–156 | C36H48N6O3· 4oxalate·4H2Oi | 0.0033 ± 0.0006 | 0.022 ± 0.0012 | n.d. | n.d. |
Calcd 5.28 H, found 5.87.
Calcd 13.08 N, found 11.93.
Calcd 13.58 N, found 12.58.
Calcd 6.25 H, found 5.45.
Calcd 17.33 N, found 16.73.
Calcd 6.97 H, found 6.17.
Calcd 13.04 N, found 12.26.
Calcd 16.53 N, found 18.01.
Calcd 8.04 N, found 6.84.
R″ = Et.
R″ = (CH2)6CN.
n.d. = not determined.
