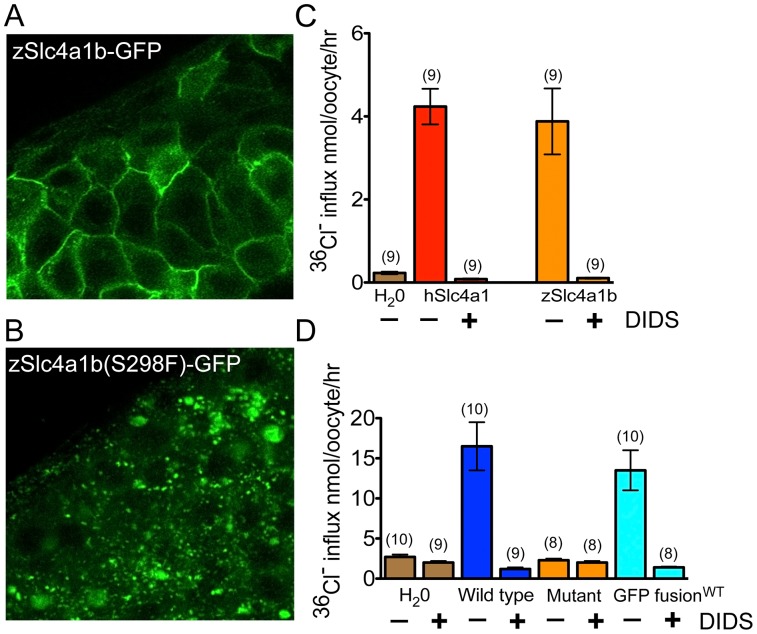
Figure 5. Mutant Slc4a1b is mislocalized and fails to transport chloride.
(A) Confocal images of zebrafish embryos transiently expressing wildtype Slc4a1b–GFP. mRNA was injected into one cell stage embryos. Cells along the anterior dorsal aspect of embryos were imaged at 48 hpf. Wildtype protein is localized to the plasma membrane. B) Confocal images of zebrafish embryos expressing mutant Slc4a1b(S298F)-GFP. Slc4(S298F)-GFP is present in intracellular vesicles, with complete loss of plasma membrane localization. (C) Chloride influx into Xenopus laevis oocytes expressing either zebrafish Slc4a1b (zSlc4a1b) or the closest human homolog, SLC4A1 (hSlc4a1). Zebrafish Slc4a1b transports radiolabeled chloride across oocyte membranes as efficiently as its nearest human homolog. This activity is blocked by DIDS (200 µM), an inhibitor of Slc4 family-mediated anion exchangers. (D) Comparison of chloride influx into Xenopus laevis oocytes expressing wildtype or mutant (S298F) zSlc4a1b, or wildtype zSlc4a1b-GFP. zSlc4a1(S298F), the persephone gene product, exhibits complete loss of chloride influx activity. Notably, GFP-tagged wildtype slc4a1b exhibits no significant reduction of chloride transport activity. (n indicated above each sample; Error bars = SEM).