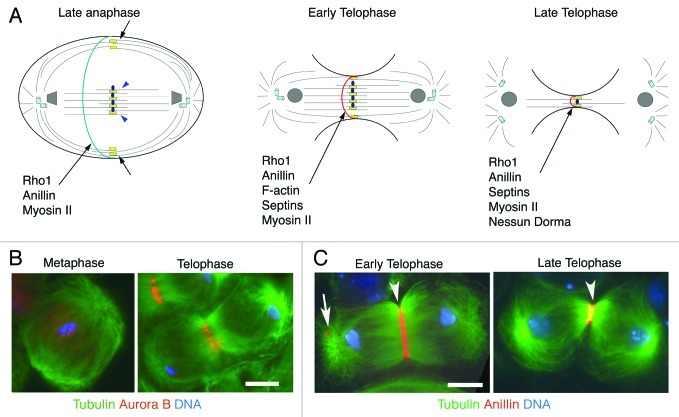
Figure 2. Cytokinesis in Drosophila spermatocytes. (A) Schematic representation of different stages of male meiotic cytokinesis. During late anaphase the central spindle is made up of two distinct populations of microtubule bundles, the “peripheral” astral microtubules (short black arrows) and the “interior” central spindle microtubules (blue arrowheads). The kinesin Pavarotti (Pav) is enriched at the central spindle midzone and associates with both populations of microtubule bundles (yellow rectangles). The microtubule plus-end stabilizing protein Orbit specifically localizes to the interior central spindle microtubules but does not associate with the peripheral astral microtubules (blue ovals). Other microtubule associated proteins, required for central spindle formation, such as Fascetto, KLP3A, KLP67A and the Chromosomal Passenger Complex (CPC) are not depicted. Rho1, Anillin and Myosin II concentrate in a narrow cortical ring during late anaphase, before the recruitment of F-actin. During early- and mid-telophase, peripheral astral microtubule bundles merge with interior microtubule bundles. At this stage Rho1, Anillin, F-actin, Septins and Myosin II colocalize in the contractile rings. Nessun Dorma is recruited to the cleavage furrow during late telophase; this protein, like Septins, Anillin and Myosin II, is a ring canal component after the completion of cytokinesis. (B) Localization of the CPC protein Aurora B in dividing spermatocytes. Primary spermatocytes were stained for tubulin (green), Aurora B (red) and DNA (blue). Note that Aurora B is enriched at metaphase centromeres and concentrates at the central spindle midzone during telophase. (C) Localization of Anillin in early telophase spermatocytes. Spermatocytes were stained for tubulin (green), Anillin (red) and DNA (blue). Arrowhead points to the contractile ring, arrow points to a ring canal. Bars, 10 μm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.