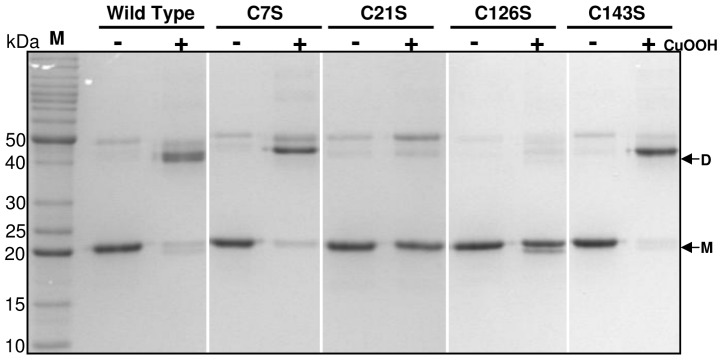
Figure 10. Investigation of intermolecular disulfide bond formation on OhrR by nonreducing SDS-PAGE.
Purified OhrR variants were reduced by DTT treatment. Samples of reduced proteins were either untreated (−) or treated (+) with 100 µM cumene hydroperoxide (CuOOH) for 30 min before alkylation. The NEM-alkylated proteins were separated by nonreducing SDS-PAGE, stained with Coomassie Brilliant Blue and subsequently destained. The monomeric (M) and dimeric (D) forms of OhrR are indicated by arrows. M represents the protein molecular mass standards.