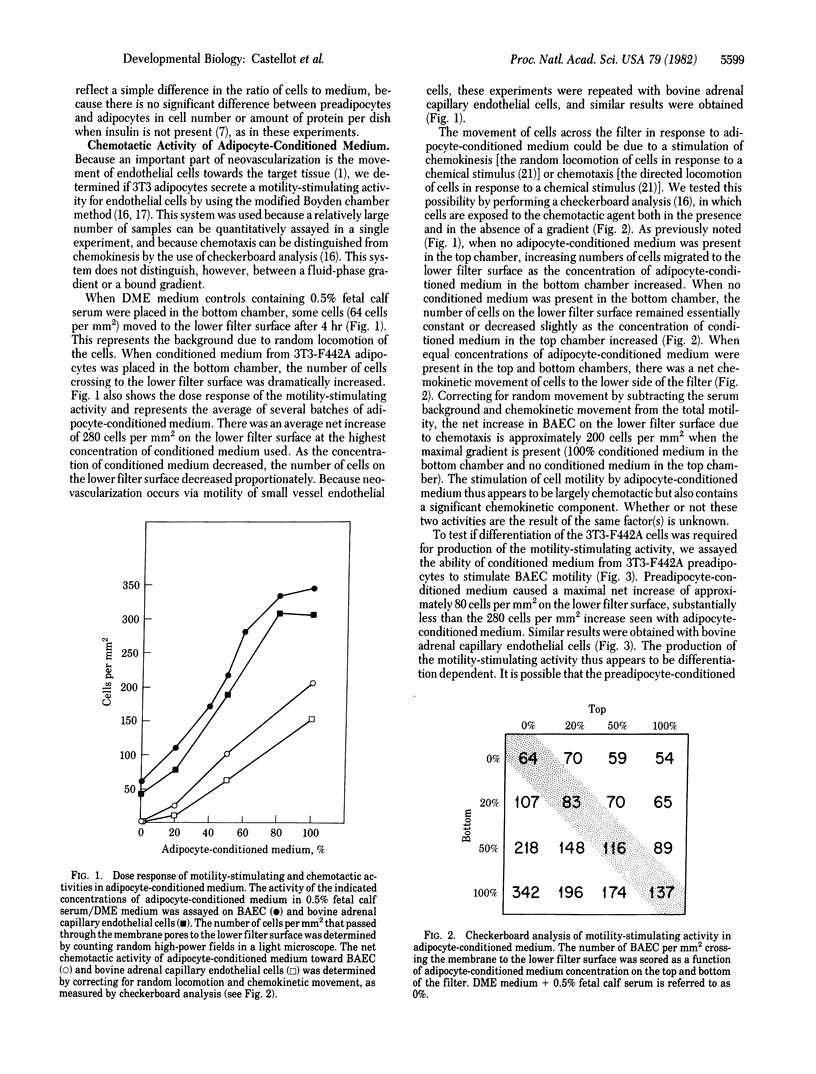
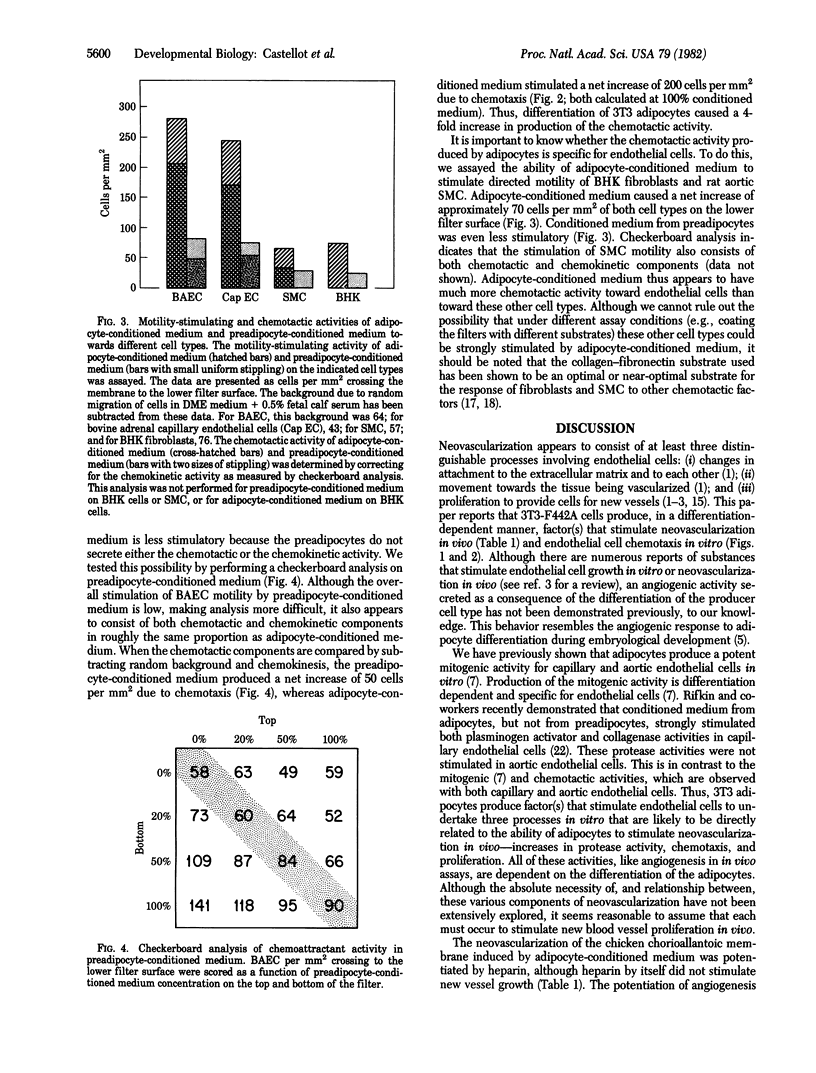
Abstract
3T3 cells that have undergone adipose differentiation in vitro secrete factor(s) that stimulate angiogenesis (neovascularization) in vivo. When medium containing 0.5% fetal calf serum was conditioned by 3T3-F442A adipocytes, it stimulated angiogenesis when placed on the chicken chorioallantoic membrane. Control medium or medium conditioned by preadipocytes did not stimulate angiogenesis, even at much higher doses. Thus, the production of the angiogenic activity is strongly dependent upon differentiation of the adipocytes. The degree of the angiogenic response to adipocyte-conditioned medium was potentiated by heparin; heparin added to unconditioned medium or to preadipocyte-conditioned medium was not angiogenic. The adipocyte-conditioned medium also strongly stimulated, in a differentiation-dependent fashion, the motility of aortic and capillary endothelial cells in a modified Boyden chamber assay. Checkerboard analysis cells indicated that 75% of the motility-stimulating activity was chemotactic in nature. The chemotactic activity has an apparent specificity for endothelial cells, in that chemotaxis of smooth muscle cells and fibroblasts was stimulated to a much lesser extent. These results, in conjunction with our previous demonstration of an endothelial cell mitogen produced by 3T3 adipocytes [Castellot, J. J., Jr., Karnovsky, M. J. & Spiegelman, B. M. (1980) Proc. Natl. Acad. Sci. USA 77, 6007-6011] indicate that the differentiation of these cells is closely linked to the production of factors that stimulate angiogenesis in vivo and growth and chemotaxis of endothelial cells in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Karnovsky M. J., Spiegelman B. M. Potent stimulation of vascular endothelial cell growth by differentiated 3T3 adipocytes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6007–6011. doi: 10.1073/pnas.77.10.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974 Aug;34(8):2109–2113. [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6107–6109. doi: 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Hoffman H. Endothelium stimulating factor from Walker carcinoma cells. Relation to tumor angiogenic factor. Exp Cell Res. 1979 Mar 1;119(1):181–190. doi: 10.1016/0014-4827(79)90347-1. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Taylor S., Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982 May 27;297(5864):307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


