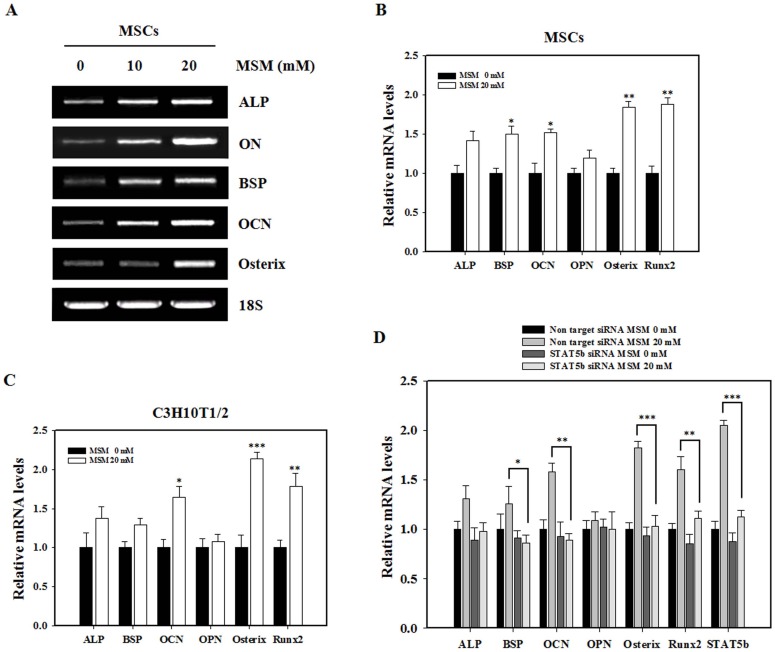
Figure 7. Involvement of STAT5b in MSM-induced osteogenic marker genes in MSCs.
(A) Bone marrow mesenchymal stem cells were cultured in the osteogenic medium at 5 days for ALP, 14 days for osteonectin (ON) and bone sialoprotein (BSP), and 21 days for osteocalcin (OCN) and osterix mRNA expression after the treatment with various concentrations (0, 10 and 20 mM) of MSM. RT-PCR was performed using the cDNA and primers for ALP, ON, BSP, OCN, osterix and 18S. Total RNA was isolated from the MSCs using an RNeasy kit. 18S was used as a control. (B) Bone marrow Mesenchymal stem cells and (C) C3H10T1/2 cells were cultured in osteogenic medium at 5 days for ALP and Runx2, 14 days for OPN and BSP, and 21 days for OCN and osterix mRNA expression after the treatment with 20 mM MSM. After culture, real-time PCR was performed. (D) Osteogenic differentiation marker genes (ALP, BSP, OCN, OPN, Osterix and Runx2) and STAT5b gene expression was analyzed at day 5, 14 and 21 after MSM treatment in C3H10T1/2 cells transfected with STAT5b siRNA or non-target siRNA. The effect of STAT5b knockdown on osteogenic marker genes was analyzed by real-time PCR. GAPDH was used as the internal control. The relative levels of mRNA were determined using densitometric analysis and normalized to the amount of GAPDH. Data shown are representative of three independent experiments. Asterisks indicate a statistically significant increase by t-test (*p<0.05, **p<0.01, ***p<0.001).