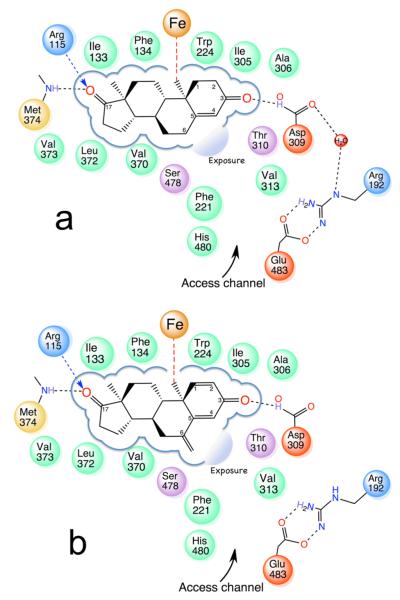
Figure 2.
Design considerations for the new inhibitors derived from the binding interactions and exposure of the ligands to the enzyme interaction spaces: (a) ASD; (b) EXM. In (a) and (b) derived from the X-ray structures, the residues lining the binding pocket making hydrophobic and hydrogen-bonding contacts are shown (hydrophobic, green; acidic, red; basic, blue; polar, purple; sulfur-containing, yellow). Exposure at the C4 and C6 positions of the steroid to the access channel opening is indicated. Also shown schematically in (a) is a water molecule trapped between Asp309 and Arg192 side chains, postulated to have a role in the proton relay network and enolization of 3-keto.6